ご利用について
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of Wilms tumor and other childhood kidney tumors. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
CONTENTS
- General Information About Childhood Kidney Tumors
-
Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2010, childhood cancer mortality decreased by more than 50%.[ 1 ] For children younger than 15 years with Wilms tumor, the 5-year survival rate has increased over the same time from 74% to 88%.[ 1 ] Childhood and adolescent cancer survivors require close monitoring because cancer therapy side effects may persist or develop months or years after treatment. (Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors.)
Childhood kidney cancers account for about 7% of all childhood cancers. Most childhood kidney cancers are Wilms tumor, but in the 15- to 19-year age group, most tumors are renal cell carcinoma. Wilms tumor can affect one kidney (unilateral) or both kidneys (bilateral). Less common types of childhood kidney tumors include rhabdoid tumors, clear cell sarcoma, congenital mesoblastic nephroma, Ewing sarcoma of the kidney, primary renal myoepithelial carcinoma, cystic partially differentiated nephroblastoma, multilocular cystic nephroma, primary renal synovial sarcoma, and anaplastic sarcoma. Nephroblastomatosis of the kidney is a type of nonmalignant neoplasia.[ 2 ][ 3 ]
参考文献- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014.[PUBMED Abstract]
- Ahmed HU, Arya M, Levitt G, et al.: Part I: Primary malignant non-Wilms' renal tumours in children. Lancet Oncol 8 (8): 730-7, 2007.[PUBMED Abstract]
- Ahmed HU, Arya M, Levitt G, et al.: Part II: Treatment of primary malignant non-Wilms' renal tumours in children. Lancet Oncol 8 (9): 842-8, 2007.[PUBMED Abstract]
- Wilms Tumor
-
Incidence of Wilms Tumor
Wilms tumor is the most frequent tumor of the kidney in infants and children. The incidence of Wilms tumor is 8.2 cases for every 1 million children younger than 15 years, or one case per 10,000 infants.[ 1 ] Approximately 650 cases of Wilms tumor are diagnosed in the United States each year. The incidence is substantially lower in Asians.
The male to female ratio in unilateral cases of Wilms tumor is 0.92 to 1.00, but in bilateral cases, it is 0.60 to 1.00. The mean age at diagnosis is 44 months in unilateral cases and 31 months in bilateral cases of Wilms tumor.[ 2 ][ 3 ] About 10% of children with Wilms tumor have an associated congenital malformation syndrome.[ 4 ]
Syndromes and Other Conditions Associated With Wilms Tumor
Wilms tumor typically develops in otherwise healthy children without any predisposition to developing cancer; however, approximately 10% of children with Wilms tumor have been reported to have a congenital anomaly.[ 4 ][ 5 ] In patients with congenital anomalies and Wilms tumor, nephrogenic rests have been reported in 60% of cases.[ 6 ] Of 295 consecutive patients with Wilms tumor seen at the Institut Curie in Paris, 52 (17.6%) had anomalies or syndromes, 43 of which were considered major, and 14 of which were genetically proven tumor predisposition syndromes.[ 7 ]
Children with Wilms tumor may have associated hemihyperplasia and urinary tract anomalies, including cryptorchidism and hypospadias. Children may have recognizable phenotypic syndromes such as overgrowth, aniridia, genetic malformations, and others. These syndromes have provided clues to the genetic basis of the disease. The phenotypic syndromes and other conditions have been grouped into overgrowth and non-overgrowth categories (refer to Table 1). Overgrowth syndromes and conditions are the result of excessive prenatal and postnatal somatic growth.[ 8 ][ 9 ]
It is important to recognize that the absolute risk of Wilms tumor varies with the underlying condition or anomaly. For example, most patients with hemihyperplasia will not develop Wilms tumor.
Table 1. Syndromes and Conditions Associated With Wilms Tumora Syndrome/Condition Gene Overgrowth Phenotype Non-Overgrowth Phenotype High Risk of Wilms Tumor (>20%) CLOVES = congenital lipomatous overgrowth, vascular malformations, epidermal nevi, and skeletal/spinal abnormalities; MULIBREY = distinctive abnormalities of the (MU)scles, (LI)ver, (BR)ain, and (EY)es; WAGR = Wilms tumor, aniridia, genitourinary anomaly, and mental retardation. aAdapted from Treger et al.[ 10 ] WAGR syndrome WT1 deletion X Denys-Drash syndrome WT1 missense mutation X Perlman syndrome DIS3L2 mutation X Fanconi anemia with biallelic mutations in BRCA2 (FANCD1) or PALB2 (FANCN) BRCA2, PALB2 X Premature chromatid separation/mosaic variegated aneuploidy Biallelic BUB1B or TRIP13 mutation X Moderate Risk of Wilms Tumor (5%–20%) Frasier syndrome WT1 intron 9 splice mutation X Beckwith-Wiedemann syndrome Uniparental disomy or H19 epimutation X Simpson-Golabi-Behmel syndrome GPC3 mutation X Low Risk of Wilms Tumor (<5%) Bloom syndrome Biallelic BLM mutation X DICER1 syndrome DICER1 mutation X Li-Fraumeni syndrome TP53, CHEK2 X Isolated hemihyperplasia X Hyperparathyroidism-jaw tumor syndrome CDC73 (also known as HRPT2) mutation X MULIBREY nanism syndrome TRIM37 mutation X PIK3CA-related segmental overgrowth including CLOVES syndrome PIK3CA mutation X 9q22.3 microdeletion syndrome 9q22.3 X Sotos syndrome NSD1 X Familial Wilms tumor FWT1 X FWT2 Genitourinary anomalies WT1 X Sporadic aniridia WT1 X Trisomy 18 X For information about the genes associated with Wilms tumor, including WT1 and WT2, refer to the Genomics of Wilms Tumor section of this summary.
Syndromic causes of Wilms tumor
WT1-related syndromes include the following:
WT2-related syndromes include the following:
-
Beckwith-Wiedemann syndrome. Beckwith-Wiedemann syndrome is an overgrowth syndrome characterized by asymmetric growth of one or more parts of the body, large tongue, omphalocele or umbilical hernia at birth, creases or pits in the skin near the ears, kidney abnormalities, and hypoglycemia (in neonates). It is also characterized by the development of Wilms tumor, rhabdomyosarcoma, and hepatoblastoma in the first decade of life. Approximately 15% of children with Beckwith-Wiedemann syndrome will have bilateral tumors.[
20
]
Beckwith-Wiedemann syndrome is caused by altered expression of two gene clusters involved in growth control and cell-cycle progression regulated by two independent imprinting control regions (ICR1 [termed telomeric ICR] and ICR2 [termed centromeric ICR]) at chromosome 11p15.5. The two ICRs are characterized by differential methylation of maternal and paternal alleles. A variety of molecular mechanisms are implicated in Beckwith-Wiedemann syndrome pathogenesis, leading to unbalanced expression of imprinted genes within these two domains. Tumor predisposition results primarily from dysregulation at the telomeric domain of 11p15 (ICR1 gain of methylation [ICR1-GoM] and paternal uniparental disomy [UPD]) rather than at the centromeric domain of 11p15 (ICR2 loss of methylation [ICR2-LoM] and CDKN1C mutation).[ 21 ] Approximately 15% of cases with clear-cut phenotypes have no molecular defects established so far.[ 22 ][ 23 ]
The molecular subtypes of the syndrome predispose patients to the development of different tumor histotypes.[ 24 ][ 25 ][ 26 ]
The prevalence of Beckwith-Wiedemann syndrome is about 1% of children with Wilms tumor.[ 20 ][ 27 ][ 28 ][ 29 ] Approximately 10% of Beckwith-Wiedemann syndrome patients will develop Wilms tumor.[ 21 ] Beckwith-Wiedemann syndrome patients with hemihyperplasia have a fourfold increased tumor risk over that of Beckwith-Wiedemann syndrome patients without hemihyperplasia.[ 30 ] (Refer to the Genomics of Wilms Tumor section of this summary for more information.)
Other syndromic causes of Wilms tumor include the following:
-
Perlman syndrome. Perlman syndrome—a rare, autosomal, recessively inherited, congenital overgrowth syndrome—is characterized by fetal gigantism, renal dysplasia and nephroblastomatosis, islet cell hypertrophy, multiple congenital anomalies, and mental retardation. Survivors have a high risk of developing Wilms tumor (75%).[
31
]
Germline inactivating mutations in DIS3L2 on chromosome 2q37 are associated with Perlman syndrome. Preliminary data suggest that DIS3L2 plays a role in normal kidney development and in a subset of sporadic Wilms tumor cases.[ 32 ]
-
Simpson-Golabi-Behmel syndrome. Simpson-Golabi-Behmel syndrome is characterized by macroglossia, macrosomia, renal and skeletal abnormalities, and increased risk of embryonal cancers.
The syndrome is caused by mutations or deletions in glypican genes GPC3 and GPC4, and these genetic aberrations are believed to enhance the risk of Wilms tumor (8%).[ 33 ]
-
CLOVES syndrome. This syndrome is characterized by the following:
- Congenital Lipomatous Overgrowth.
- Vascular malformations.
- Epidermal nevi.
- Skeletal/spinal abnormalities.
This syndrome results from postzygotic, somatic mutations in PIK3CA, which may involve large or small regions of the child.[ 34 ]
-
Sotos syndrome. Sotos syndrome is characterized by cerebral gigantism and learning disability, ranging from mild to severe. Sotos syndrome is associated with behavioral problems, congenital cardiac anomalies, neonatal jaundice, and renal anomalies such as Wilms tumor, scoliosis, and seizures.
NSD1 is the only gene in which mutations are known to cause Sotos syndrome.[ 35 ]
-
9q22.3 microdeletion syndrome. 9q22.3 microdeletion syndrome is characterized by craniofacial abnormalities, metopic craniosynostosis, hydrocephalus, macrosomia, and learning disabilities.
Three patients presented with Wilms tumor in addition to a constitutional 9q22.3 microdeletion and dysmorphic/overgrowth syndrome. Although the size of the deletions was variable, all encompassed the PTCH1 gene.[ 36 ]
-
Bloom syndrome. Bloom syndrome is characterized by short stature and being thinner than other family members, sun-sensitive skin changes, and an increased risk of Wilms tumor.
BLM is the only gene in which mutations are known to cause Bloom syndrome.[ 37 ]
-
Li-Fraumeni syndrome. Li-Fraumeni syndrome is a rare disorder that greatly increases the risk of developing several types of cancer, particularly in children and young adults.
The cancers most often associated with Li-Fraumeni syndrome include breast cancer, osteosarcoma, soft tissue sarcoma, brain tumor, leukemia, adrenocortical carcinoma, and Wilms tumor.
The TP53 gene mutation is present in most families with Li-Fraumeni syndrome. The CHEK2 gene mutation is also known to cause Li-Fraumeni syndrome.[ 38 ]
- Alagille syndrome. Alagille syndrome includes congenital cardiopathy; facial dysmorphology; and vertebral, ocular, and renal abnormalities. It has been reported with Wilms tumor in two cases with identified mutations.[ 39 ]
-
Bohring-Opitz syndrome. Bohring-Opitz syndrome is a rare genetic condition characterized by distinctive facial features, variable microcephaly, hypertrichosis, nevus flammeus, severe myopia, unusual posture, severe intellectual disability, and feeding issues.
The syndrome is associated with ASXL1 mutations and an estimated 7% incidence of Wilms tumor.[ 40 ]
Nonsyndromic causes of Wilms tumor
Nonsyndromic causes of Wilms tumor include the following:
-
Familial Wilms tumor. Despite the number of genes that appear to be involved in the development of
Wilms tumor, familial Wilms tumor is uncommon, with approximately 2% of patients
having a positive family history for Wilms tumor. Siblings of children with Wilms tumor have a less-than-1% chance of developing Wilms tumor.[
41
][
42
][
43
] The risk of Wilms
tumor among offspring of persons who have had unilateral (sporadic)
tumors is less than 2%.[
44
]
Two familial Wilms tumor genes have been localized to FWT1 (17q12-q21) and FWT2 (19q13.4).[ 45 ] [ 46 ][ 47 ] Occasional Wilms tumor families have a germline mutation in WT1. In these families, most, but not all, family members have genitourinary tract malformations.[ 48 ][ 49 ]
Inactivating mutations in CTR9 have been identified in 3 of 35 Wilms tumor families. CTR9 is located at 11p15.3 and is a key component of the polymerase-associated factor 1 (PAF1) complex, which has multiple roles in RNA polymerase II regulation and transcriptional elongation and is implicated in embryonic organogenesis.[ 50 ]
A few families with familial Wilms tumor have germline microdeletion or microinsertion mutations in the H19 region of 11p15.3 that result in hypermethylation of the site.[ 51 ]
- Sporadic aniridia. Sporadic aniridia may result from small germline deletions of one copy of the PAX6 gene that includes part or all of the adjacent WT1 gene but does not result in genitourinary abnormalities or retardation (i.e., not obviously WAGR syndrome). Therefore, many patients with sporadic aniridia develop Wilms tumor and are candidates for genetic testing. The relative risk of Wilms tumor in sporadic aniridia is 67-fold.[ 52 ] About half of individuals with sporadic aniridia and PAX6 and WT1 deletions develop Wilms tumor.[ 53 ]
- Constitutional 11p15 abnormalities. Constitutional 11p15 abnormalities have been identified in lymphocyte DNA of 13 of 437 individuals (3%) with sporadic Wilms tumor without features of growth disorders, including 12% of bilateral cases. All were de novo abnormalities and appeared to be postzygotic, except for one novel microdeletion in a child whose mother had the mutation and was not affected; however, a subsequently born brother with the microdeletion had Beckwith-Wiedemann syndrome. This suggests that constitutional 11p15 analysis should be considered in all individuals with Wilms tumor.[ 51 ]
-
Isolated hemihyperplasia. Hemihyperplasia is an asymmetric overgrowth of one or more body parts and is associated with Wilms tumor. It can also be associated with other predisposition syndromes such as Beckwith-Wiedemann syndrome. Clinical signs may not be very evident, and hemihyperplasia may be noted after tumor diagnosis.
The overall Wilms tumor incidence was 5.9% in a study of 168 patients with isolated hemihyperplasia, although this result may have been affected by ascertainment bias.[ 54 ] The prevalence is about 2.5% of children with Wilms tumor.[ 27 ][ 54 ]
- Trisomy 18.[ 55 ]
- Fanconi anemia with biallelic mutations in BRCA2 (FANCD1) or PALB2 (FANCN). BRCA2 and PALB2 play central roles in homologous recombination DNA repair. Biallelic mutations in either BRCA2 or PALB2 lead to Fanconi anemia and to increased risks of selected childhood cancers, including Wilms tumor.[ 56 ][ 57 ][ 58 ]
Genomics of Wilms Tumor
Wilms tumors, similar to other pediatric embryonal neoplasms, typically arise after a limited number of genetic aberrations. One study performed genome-wide sequencing, mRNA and miRNA expression, DNA copy number, and methylation analysis on 117 Wilms tumors followed by targeted sequencing of 651 Wilms tumors.[ 59 ] The tumors were selected for either favorable histology (FH) Wilms that had relapsed or those with diffuse anaplasia. The study showed the following:[ 59 ]
- Wilms tumors commonly arise through more than one genetic event.
- Wilms tumors show differences in gene expression and methylation patterns with different genetic aberrations.
- Wilms tumors have a large number of candidate driver genes, most of which are mutated in less than 5% of Wilms tumors.
- Wilms tumors have recurrent mutations in genes with common functions, with most involved in either early renal development or epigenetic regulation (e.g., chromatin modifications, transcription elongation, and miRNA).
Approximately one-third of Wilms tumor cases involve mutations in WT1, CTNNB1, or WTX.[ 60 ][ 61 ] Another subset of Wilms tumor cases results from mutations in miRNA processing genes (miRNAPG), including DROSHA, DGCR8, DICER1, and XPO5.[ 62 ][ 63 ][ 64 ][ 65 ] Other genes critical for early renal development that are recurrently mutated in Wilms tumor include SIX1 and SIX2 (transcription factors that play key roles in early renal development),[ 62 ][ 63 ] EP300, CREBBP, and MYCN.[ 59 ] Of the mutations in Wilms tumors, 30% to 50% appear to converge on the process of transcriptional elongation in renal development and include the genes MLLT1, BCOR, MAP3K4, BRD7, and HDAC4.[ 59 ] Anaplastic Wilms tumor is characterized by the presence of TP53 mutations.
Elevated rates of Wilms tumor are observed in patients with a number of genetic disorders, including WAGR (Wilms tumor, aniridia, genitourinary anomalies, and mental retardation) syndrome, Beckwith-Wiedemann syndrome, hemihypertrophy, Denys-Drash syndrome, and Perlman syndrome.[ 66 ] Other genetic causes that have been observed in familial Wilms tumor cases include germline mutations in REST and CTR9.[ 50 ][ 67 ]
The genomic and genetic characteristics of Wilms tumor are summarized below.
WT1 gene
The WT1 gene is located on the short arm of chromosome 11 (11p13). WT1 is a transcription factor that is required for normal genitourinary development and is important for differentiation of the renal blastema.[ 68 ] WT1 mutations are observed in 10% to 20% of cases of sporadic Wilms tumor.[ 60 ][ 68 ][ 69 ]
Wilms tumor with a WT1 mutation is characterized by the following:
- Evidence of WNT pathway activation by activating mutations in the CTNNB1 gene is common.[ 69 ][ 70 ][ 71 ]
- Loss of heterozygosity (LOH) at 11p15 is commonly observed, as paternal uniparental disomy for chromosome 11 represents a common mechanism for losing the remaining normal WT1 allele.[ 69 ][ 72 ]
- Nephrogenic rests are benign foci of embryonal kidney cells that abnormally persist into postnatal life. Intralobar nephrogenic rests occur in approximately 20% of Wilms tumor cases. They are observed at high rates in cases with genetic syndromes that have WT1 mutations such as WAGR and Denys-Drash syndromes.[ 73 ] Intralobar nephrogenic rests are also observed in cases with sporadic WT1 and MLLT1 mutations.[ 74 ][ 75 ]
- WT1 germline mutations are uncommon (2%–4%) in nonsyndromic Wilms tumor.[ 49 ][ 76 ]
- WT1 mutations and 11p15 LOH were associated with relapse in patients with very low-risk Wilms tumor in one study of 56 patients who did not receive chemotherapy.[ 77 ] These findings need validation but may provide biomarkers for stratifying patients in the future.
Germline WT1 mutations are more common in children with Wilms tumor and one of the following:
Syndromic conditions with germline WT1 mutations include WAGR syndrome, Denys-Drash syndrome,[ 17 ] and Frasier syndrome.[ 14 ]
-
WAGR syndrome. Children with WAGR syndrome are at high risk (approximately 50%) of developing Wilms tumor.[
4
] WAGR syndrome results from deletions at chromosome 11p13 that involve a set of contiguous genes that includes the WT1 and PAX6 genes.
Inactivating mutations or deletions in the PAX6 gene lead to aniridia, while deletion of WT1 confers the increased risk of Wilms tumor. Sporadic aniridia in which WT1 is not deleted is not associated with increased risk of Wilms tumor. Accordingly, children with familial aniridia, generally occurring for many generations, and without renal abnormalities, have a normal WT1 gene and are not at an increased risk of Wilms tumor.[ 27 ][ 78 ]
Wilms tumor in children with WAGR syndrome is characterized by an excess of bilateral disease, intralobar nephrogenic rests, early age at diagnosis, and stromal-predominant histology in FH tumors.[ 13 ] The mental retardation in WAGR syndrome may be secondary to deletion of other genes, including SLC1A2 or BDNF.[ 51 ]
Germline WT1 point mutations produce genetic syndromes that are characterized by nephropathy, 46XY disorder of sex development, and varying risks of Wilms tumor.[ 79 ][ 80 ]
-
Denys-Drash and Frasier syndromes. Denys-Drash syndrome is characterized by nephrotic syndrome caused by diffuse mesangial sclerosis, XY pseudohermaphroditism, and increased risk of Wilms tumor (>90%). Frasier syndrome is characterized by progressive nephropathy caused by focal segmental glomerulosclerosis, gonadoblastoma, and XY pseudohermaphroditism.
WT1 mutations in Denys-Drash syndrome are most often missense mutations in exons 8 and 9, which code for the DNA binding region of WT1.[ 17 ] By contrast, WT1 mutations in Frasier syndrome typically occur in intron 9 at the KTS site, and create an alternative splicing variant, thereby preventing production of the usually more abundant WT1 +KTS isoform.[ 19 ]
Studies evaluating genotype/phenotype correlations of WT1 mutations have shown that the risk of Wilms tumor is highest for truncating mutations (14 of 17 cases, 82%) and lower for missense mutations (27 of 67 cases, 42%). The risk is lowest for KTS splice site mutations (1 of 27 cases, 4%).[ 79 ][ 80 ] Bilateral Wilms tumor was more common in cases with WT1-truncating mutations (9 of 14 cases) than in cases with WT1 missense mutations (3 of 27 cases).[ 79 ][ 80 ] These genomic studies confirm previous estimates of elevated risk of Wilms tumor for children with Denys-Drash syndrome and low risk of Wilms tumor for children with Frasier syndrome.
Late effects associated with WAGR syndrome and Wilms tumor include the following:
- Children with WAGR syndrome or other germline WT1 mutations are monitored throughout their lives because they are at increased risk of developing hypertension, nephropathy, and renal failure.[ 81 ]
- Patients with Wilms tumor and aniridia without genitourinary abnormalities are at lower risk but are monitored for nephropathy or renal failure.[ 82 ]
- Children with Wilms tumor and any genitourinary anomalies are also at increased risk of late renal failure and are monitored. Features associated with germline WT1 mutations that increase the risk of developing renal failure include the following:[
81
]
- Stromal predominant histology.
- Bilateral disease.
- Intralobar nephrogenic rests.
- Wilms tumor diagnosed before age 2 years.
(Refer to the Late effects after Wilms tumor therapy section of the PDQ summary on Wilms Tumor and Other Childhood Kidney Tumors Treatment for more information about the late effects associated with Wilms tumor.)
CTNNB1 gene
CTNNB1 is the most commonly mutated gene in Wilms tumor, reported to occur in 15% of patients with Wilms tumor.[ 59 ][ 61 ][ 69 ][ 71 ][ 83 ] These CTNNB1 mutations result in activation of the WNT pathway, which plays a prominent role in the developing kidney.[ 84 ] CTNNB1 mutations commonly occur with WT1 mutations, and most cases of Wilms tumor with WT1 mutations have a concurrent CTNNB1 mutation.[ 69 ][ 71 ][ 83 ] Activation of beta-catenin in the presence of intact WT1 protein appears to be inadequate to promote tumor development because CTNNB1 mutations are rarely found in the absence of a WT1 or WTX mutation, except when associated with a MLLT1 mutation.[ 61 ][ 85 ] CTNNB1 mutations appear to be late events in Wilms tumor development because they are found in tumors but not in nephrogenic rests.[ 74 ]
WTX gene on the X chromosome
WTX, which is also called AMER1, is located on the X chromosome at Xq11.1. It is altered in 15% to 20% of Wilms tumor cases.[ 60 ][ 61 ][ 69 ][ 86 ][ 87 ] Germline mutations in WTX cause an X-linked sclerosing bone dysplasia, osteopathia striata congenita with cranial sclerosis (MIM300373).[ 88 ] Despite having germline WTX mutations, individuals with osteopathia striata congenita are not predisposed to tumor development.[ 88 ] The WTX protein appears to be involved in both the degradation of beta-catenin and in the intracellular distribution of APC protein.[ 85 ][ 89 ] WTX is most commonly altered by deletions involving part or all of the WTX gene, with deleterious point mutations occurring less commonly.[ 60 ][ 69 ][ 86 ] Most Wilms tumor cases with WTX alterations have epigenetic 11p15 abnormalities.[ 69 ]
WTX alterations are equally distributed between males and females, and WTX inactivation has no apparent effect on clinical presentation or prognosis.[ 60 ]
Imprinting cluster regions (ICRs) on chromosome 11p15 (WT2) and Beckwith-Wiedemann syndrome
A second Wilms tumor locus, WT2, maps to an imprinted region of chromosome 11p15.5; when it is a germline mutation, it causes Beckwith-Wiedemann syndrome. About 3% of children with Wilms tumor have germline epigenetic or genetic changes at the 11p15.5 growth regulatory locus without any clinical manifestations of overgrowth. Like children with Beckwith-Wiedemann syndrome, these children have an increased incidence of bilateral Wilms tumor or familial Wilms tumor.[ 51 ]
Approximately one-fifth of patients with Beckwith-Wiedemann syndrome who develop Wilms tumor present with bilateral disease, and metachronous bilateral disease is also observed.[ 27 ][ 28 ][ 29 ] The prevalence of Beckwith-Wiedemann syndrome is about 1% among children with Wilms tumor reported to the National Wilms Tumor Study (NWTS).[ 2 ][ 29 ]
Approximately 80% of patients with Beckwith-Wiedemann syndrome have a molecular defect of the 11p15 domain.[ 90 ] Various molecular mechanisms underlying Beckwith-Wiedemann syndrome have been identified. Some of these abnormalities are genetic (germline mutations of the maternal allele of CDKN1C, paternal uniparental isodisomy of 11p15, or duplication of part of the 11p15 domain) but are more frequently epigenetic (loss of methylation of the maternal ICR2/KvDMR1 or gain of methylation of the maternal ICR1).[ 51 ][ 91 ]
Several candidate genes at the WT2 locus comprise the two independent imprinted domains IGF2/H19 and KIP2/LIT1.[ 91 ] LOH, which exclusively affects the maternal chromosome, has the effect of upregulating paternally active genes and silencing maternally active ones. A loss or switch of the imprint for genes (change in methylation status) in this region has also been frequently observed and results in the same functional aberrations.[ 51 ][ 90 ][ 91 ]
A relationship between epigenotype and phenotype has been shown in Beckwith-Wiedemann syndrome, with a different rate of cancer in Beckwith-Wiedemann syndrome according to the type of alteration of the 11p15 region.[ 92 ]
The following four main molecular subtypes of Beckwith-Wiedemann syndrome are characterized by specific genotype-phenotype correlations:
- ICR1 gain of methylation (ICR1-GoM). Five percent to 10% of cases are caused by telomeric ICR1-GoM, which causes both biallelic expression of the IGF2 gene (normally expressed by the paternal allele only) and reduced expression of the oncosuppressor H19 gene. The incidence of Wilms tumor is 22.8%.[ 93 ]
- ICR2 loss of methylation (ICR2-LoM). Fifty percent of cases with Beckwith-Wiedemann syndrome are caused by ICR2-LoM, resulting in reduced expression of the CDKN1C gene, normally expressed by the maternal chromosome only. Tumor incidence is very low (2.5%).[ 93 ]
- Uniparental disomy (UPD). Altered expression at both imprinted gene clusters is observed in mosaic UPD of chromosome 11p15.5, accounting for 20% to 25% of the cases. The incidence of Wilms tumor is 6.2%, followed by hepatoblastoma (4.7%) and adrenal carcinoma (1.5%).[ 93 ] Fewer than 1% of cases with Beckwith-Wiedemann syndrome are caused by chromosomal rearrangements involving the 11p15 region.
- CDKN1C mutations. Maternally inheritable CDKN1C loss-of-function mutations account for approximately 5% of the cases. This type is associated with a 4.3% incidence of neuroblastoma.[ 93 ]
Other tumors such as neuroblastoma or hepatoblastoma were reported in patients with paternal 11p15 isodisomy.[ 21 ][ 25 ][ 94 ] For patients with Beckwith-Wiedemann syndrome, the relative risk of developing hepatoblastoma is 2,280 times that of the general population.[ 29 ]
Loss of imprinting or gene methylation is rarely found at other loci, supporting the specificity of loss of imprinting at 11p15.5.[ 95 ] Interestingly, Wilms tumor in Asian children, which occur at a lower incidence than in European children, is not associated with either nephrogenic rests or IGF2 loss of imprinting.[ 96 ]
Other genes and chromosomal alterations
Additional genes and chromosomal alterations that have been implicated in the pathogenesis and biology of Wilms tumor include the following:
-
1q. Gain of chromosome 1q is associated with an inferior outcome and is the single most powerful predictor of outcome. In the presence of 1q gain, neither 1p nor 16q loss is significant.[
97
][
98
] Gain of chromosome 1q is one of the most common cytogenetic abnormalities in Wilms tumor and is observed in approximately 30% of tumors.
In an analysis of FH Wilms tumor from 1,114 patients from NWTS-5 (COG-Q9401/NCT00002611), 28% of the tumors displayed 1q gain.[ 97 ]
- The 8-year event-free survival (EFS) rate was 77% for patients with 1q gain and 90% for those lacking 1q gain (P < .001). Within each disease stage, 1q gain was associated with inferior EFS.
- The 8-year overall survival (OS) rate was 88% for those with 1q gain and 96% for those lacking 1q gain (P < .001). OS was significantly inferior in cases with stage I disease (P < .0015) and stage IV disease (P = .011).
-
16q and 1p. Additional tumor-suppressor or tumor-progression genes may lie on chromosomes 16q and 1p, as evidenced by LOH for these regions in 17% and 11% of Wilms tumor cases, respectively.[
99
]
- In large NWTS studies, patients with tumor-specific loss of these loci had significantly worse relapse-free survival and OS rates. Combined loss of 1p and 16q are used to select FH Wilms tumor patients for more aggressive therapy in the current Children's Oncology Group (COG) study. However, a U.K. study of more than 400 patients found no significant association between 1p deletion and poor prognosis, but a poor prognosis was associated with 16q LOH.[ 100 ]
- An Italian study of 125 patients, using treatment quite similar to that in the COG study, found significantly worse prognosis in those with 1p deletions but not 16q deletions.[ 101 ]
These conflicting results may arise from the greater prognostic significance of 1q gain described above. LOH of 16q and 1p loses significance as independent prognostic markers in the presence of 1q gain. However, in the absence of 1q gain, LOH of 16q and 1p retains their adverse prognostic impact.[ 97 ] The LOH of 16q and 1p appears to arise from complex chromosomal events that result in 1q LOH or 1q gain. The change in 1q appears to be the critical tumorigenic genetic event.[ 102 ]
-
miRNAPG. Mutations in selected miRNAPG are observed in approximately 20% of Wilms tumor cases and appear to perpetuate the progenitor state.[
59
][
62
][
63
][
64
][
65
] The products of these genes direct the maturation of miRNAs from the initial pri-miRNA transcripts to functional cytoplasmic miRNAs (refer to Figure 1).[
103
] The most commonly mutated miRNAPG is DROSHA, with a recurrent mutation (E1147K) affecting a metal-binding residue of the RNase IIIb domain, representing about 80% of DROSHA-mutated tumors. Other miRNAPG that are mutated in Wilms tumor include DGCR8, DICER1, TARBP2, DIS3L2, and XPO5. These mutations are generally mutually exclusive, and they appear to be deleterious and result in impaired expression of tumor-suppressing miRNAs. A striking sex bias was noted in mutations for DGCR8 (located on chromosome 22q11), with 38 of 43 cases (88%) arising in girls.[
62
][
63
]
Germline mutations in miRNAPG are observed for DICER1 and DIS3L2, with mutations in the former causing DICER1 syndrome and mutations in the latter causing Perlman syndrome.
- DICER1 syndrome is typically caused by inherited truncating mutations in DICER1, with tumor formation following acquisition of a missense mutation in a domain of the remaining allele of DICER1 (the RNase IIIb domain) responsible for processing miRNAs derived from the 5p arms of pre-miRNAs.[ 104 ] Tumors associated with DICER1 syndrome include pleuropulmonary blastoma, cystic nephroma, ovarian sex cord–stromal tumors, multinodular goiter, and embryonal rhabdomyosarcoma.[ 104 ] Wilms tumor is an uncommon presentation of the DICER1 syndrome. In one study, three families with DICER1 syndrome included children with Wilms tumor, with two of the Wilms tumor cases showing the typical second DICER1 mutation in the RNase IIIb domain.[ 105 ] Another study identified DICER1 mutations in 2 of 48 familial Wilms tumor families.[ 106 ] Large sequencing studies of Wilms tumor cohorts have also observed occasional cases with DICER1 mutations.[ 63 ][ 64 ]
- Perlman syndrome is a rare overgrowth disorder caused by mutations in DIS3L2, which encodes a ribonuclease that is responsible for degrading pre-let-7 miRNA.[
32
][
107
] The prognosis of Perlman syndrome is poor, with a high neonatal mortality rate. In a survey of published cases of Perlman syndrome (N = 28), in infants who survived beyond the neonatal period, approximately two-thirds developed Wilms tumor, and all patients showed developmental delay. Fetal macrosomia, ascites, and polyhydramnios are frequent manifestations.[
108
]
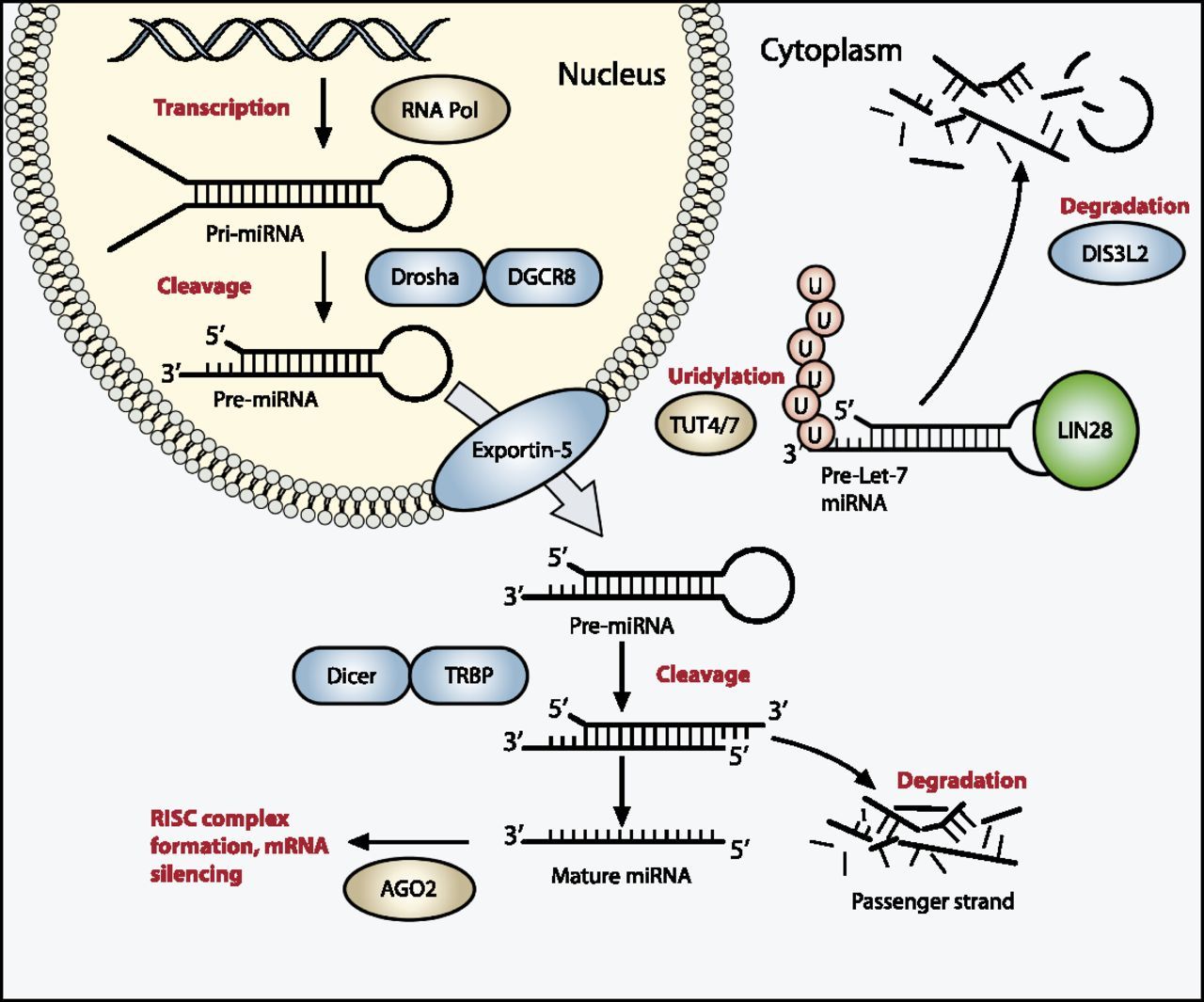
Figure 1. The miRNA processing pathway is commonly mutated in Wilms tumor. Expression of mature miRNA is initiated by RNA polymerase–mediated transcription of DNA-encoded sequences into pri-miRNA, which form a long double-stranded hairpin. This structure is then cleaved by a complex of Drosha and DGCR8 into a smaller pre-miRNA hairpin, which is exported from the nucleus and then cleaved by Dicer (an RNase) and TRBP (with specificity for dsRNA) to remove the hairpin loop and leave two single-stranded miRNAs. The functional strand binds to Argonaute (Ago2) proteins into the RNA-induced silencing complex (RISC), where it guides the complex to its target mRNA, while the nonfunctional strand is degraded. Targeting of mRNAs by this method results in mRNA silencing by mRNA cleavage, translational repression, or deadenylation. Let-7 miRNAs are a family of miRNAs highly expressed in ESCs with tumor suppressor properties. In cases in which LIN28 is overexpressed, LIN28 binds to pre-Let-7 miRNA, preventing DICER from binding and resulting in LIN28-activated polyuridylation by TUT4 or TUT7, causing reciprocal DIS3L2-mediated degradation of Let-7 pre-miRNAs. Genes involved in miRNA processing that have been associated with Wilms tumor are highlighted in blue (inactivating) and green (activating) and include DROSHA, DGCR8, XPO5 (encoding exportin-5), DICER1, TARBP2, DIS3L2, and LIN28. Copyright © 2015 Hohenstein et al.; Published by Cold Spring Harbor Laboratory Press. Genes Dev. 2015 Mar 1; 29(5): 467–482. doi: 10.1101/gad.256396.114. This article is distributed exclusively by Cold Spring Harbor Laboratory Press under a Creative Commons License (Attribution-NonCommercial 4.0 International), as described at http://creativecommons.org/licenses/by-nc/4.0/.
- SIX1 and SIX2. SIX1 and SIX2 are highly homologous transcription factors that play key roles in early renal development and are expressed in the metanephric mesenchyme, where they maintain the mesenchymal progenitor population. The frequency of SIX1 mutations is 3% to 4% in Wilms tumor, and the frequency of SIX2 mutations in Wilms tumor is 1% to 3%.[ 62 ][ 63 ] Virtually all SIX1 and SIX2 mutations are in exon 1 and result in a glutamine-to-arginine mutation at position 177. Mutations in WT1, WTX, and CTNNB1 are infrequent in cases with SIX1/SIX2 or miRNAPG mutations. Conversely, SIX1/SIX2 mutations and miRNAPG mutations tend to occur together. In Wilms tumor, SIX1 and SIX2 mutations are associated with the high-risk blastemal subtype and with the presence of undifferentiated blastema in chemotherapy-naïve samples.[ 62 ][ 63 ]
- MLLT1. Approximately 4% of Wilms tumor cases have mutations in the highly conserved YEATS domain of MLLT1 (ENL), a gene known to be involved in transcriptional elongation by RNA polymerase II during early development.[ 75 ] The mutant MLLT1 protein shows altered binding to acetylated histone tails. Patients with MLLT1-mutant tumors present at a younger age and have a high prevalence of precursor intralobar nephrogenic rests, supporting a model whereby activating MLLT1 mutations early in renal development result in the development of Wilms tumor.
-
TP53 (tumor suppressor gene). Most anaplastic Wilms tumor cases show mutations in the TP53 tumor suppressor gene.[
109
][
110
][
111
] TP53 may be useful as an unfavorable prognostic marker.[
109
][
110
]
In a study of 118 prospectively identified patients with diffuse anaplastic Wilms tumor registered on the NWTS-5 trial, 57 patients (48%) demonstrated TP53 mutations, 13 patients (11%) demonstrated TP53 segmental copy number loss without mutation, and 48 patients (41%) lacked both (wild-type TP53 [wtTP53]). All TP53 mutations were detected by sequencing alone. Patients with stage III or stage IV disease with wtTP53 had a significantly lower relapse rate and mortality rate than did patients with TP53 abnormalities (P = .00006 and P = .00007, respectively). There was no effect of TP53 status on patients with stage I or stage II tumors. In-depth analysis of a subset of 39 patients with diffuse anaplastic Wilms tumor showed that 7 patients (18%) were wtTP53. These wtTP53 tumors demonstrated gene expression evidence of p53 pathway activation. Retrospective pathology review of wtTP53 tumors revealed no or very low volume of anaplasia in six of seven tumors. These data support the key role of TP53 loss in the development of anaplasia in Wilms tumor and support its significant clinical influence in patients who have residual anaplastic disease after surgery.[ 112 ]
- FBXW7. FBXW7, a ubiquitin ligase component, is a gene that has been identified as recurrently mutated at low rates in Wilms tumor. Mutations of this gene have been associated with epithelial-type tumor histology.[ 113 ]
- 9q22.3 microdeletion syndrome. Patients with 9q22.3 microdeletion syndrome have an increased risk of Wilms tumor.[ 36 ][ 114 ] The chromosomal region with germline deletion includes PTCH1, the gene that is mutated in Gorlin syndrome (nevoid basal cell carcinoma syndrome associated with osteosarcoma). 9q22.3 microdeletion syndrome is characterized by the clinical findings of Gorlin syndrome, as well as developmental delay and/or intellectual disability, metopic craniosynostosis, obstructive hydrocephalus, prenatal and postnatal macrosomia, and seizures.[ 114 ] Five patients who presented with Wilms tumor in the context of a constitutional 9q22.3 microdeletion have been reported.[ 36 ][ 115 ][ 116 ]
- MYCN. MYCN copy number gain was observed in approximately 13% of Wilms tumor cases, and it was more common in anaplastic cases (7 of 23 cases, 30%) than in nonanaplastic cases (11.2%).[ 117 ] Activating mutations at codon 44 (p.P44L) were identified in approximately 4% of Wilms tumor cases.[ 117 ] Germline copy number gain at MYCN has been reported in a bilateral Wilms tumor case, and germline MYCN duplication was also reported for a child with prenatal bilateral nephroblastomatosis and a family history of nephroblastoma.[ 118 ]
- CTR9. Inactivating CTR9 germline mutations were identified in 4 of 36 familial Wilms tumor pedigrees.[ 50 ][ 119 ] CTR9, which is located at chromosome 11p15.3, is a key component of the polymerase-associated factor 1 complex (PAF1c), which has multiple roles in RNA polymerase II regulation and is implicated in embryonic organogenesis and maintenance of embryonic stem cell pluripotency.
- REST. Inactivating germline mutations in REST (encoding RE1-silencing transcription factor) were identified in four familial Wilms tumor pedigrees.[ 67 ] REST is a transcriptional repressor that functions in cellular differentiation and embryonic development. Most REST mutations clustered within the portion of REST encoding the DNA-binding domain, and functional analyses showed that these mutations compromise REST transcriptional repression. When screened for REST mutations, 9 of 519 individuals with Wilms tumor who had no history of relatives with the disease tested positive for the mutation; some had parents who also tested positive.[ 67 ] These observations indicate that REST is a Wilms tumor predisposition gene associated with approximately 2% of Wilms tumor.
Figure 2 summarizes the genomic landscape of a selected cohort of Wilms tumor patients selected because they experienced relapse despite showing FH.[ 75 ] The 75 FH Wilms tumor cases were clustered by unsupervised analysis of gene expression data, resulting in six clusters. Five of six MLLT1-mutant tumors with available gene expression data were in cluster 3, and two were accompanied by CTNNB1 mutations. This cluster also contained four tumors with a mutation or small segment deletion of WT1, all of which also had either a mutation of CTNNB1 or small segment deletion or mutation of WTX. It also contained a substantial number of tumors with retention of imprinting of 11p15 (including all MLLT1-mutant tumors). The miRNAPG-mutated cases clustered together and were mutually exclusive with both MLLT1 and with WT1/WTX/CTNNB1-mutated cases.
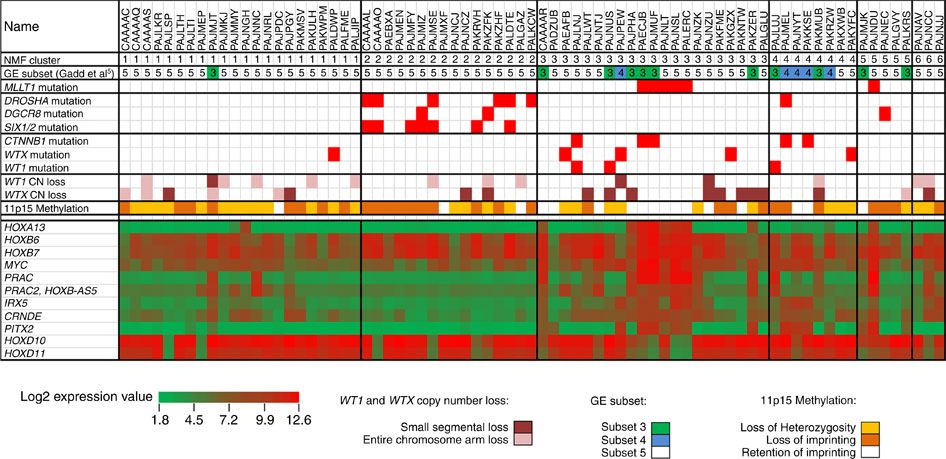
Figure 2. Unsupervised analysis of gene expression data. Non-negative Matrix Factorization (NMF) analysis of 75 FH Wilms tumor resulted in six clusters. Five of six MLLT1 mutant tumors with available gene expression data occurred in NMF cluster 3, and two were accompanied by CTNNB1 mutations. This cluster also contained a substantial number of tumors with retention of imprinting of 11p15 (including all MLLT1-mutant tumors), in contrast to other clusters, where most cases showed 11p15 loss of heterozygosity or retention of imprinting. Almost all miRNAPG-mutated cases were in NMF cluster 2, and most WT1, WTX, and CTNNB1 mutations were in NMF clusters 3 and 4. Copyright © 2015 Perlman, E. J. et al. MLLT1 YEATS domain mutations in clinically distinctive Favourable Histology wilms tumours. Nat. Commun. 6:10013 doi: 10.1038/ncomms10013 (2015). This article is distributed by Nature Publishing Group, a division of Macmillan Publishers Limited under a Creative Commons Attribution 4.0 International License, as described at http://creativecommons.org/licenses/by/4.0/. Bilateral Wilms Tumor
Approximately 5% to 10% of individuals with Wilms tumor have bilateral or multicentric tumors. The prevalence of bilateral involvement is higher in individuals with genetic predisposition syndromes than in those without predisposition syndromes. For example, in 545 cases of bilateral Wilms tumors, bona fide pathogenic germline variants were found in 22% of patients.[ 120 ] The most common predisposition variants are mutations of WT1 and 11p15 loss of imprinting.[ 20 ][ 68 ]
Bilateral Wilms tumor with WT1 mutations are associated with early presentation in pediatric patients (age 10 months vs. age 39 months for those without a mutation) and a high frequency of WT1 nonsense mutations in exon 8. Three percent of patients with bilateral Wilms tumor have affected family members.[ 121 ]
Screening Children Predisposed to Wilms Tumor
Children with a significant increased predisposition to develop Wilms tumor (e.g., most children with Beckwith-Wiedemann syndrome or other overgrowth syndromes, WAGR syndrome, Denys-Drash syndrome, sporadic aniridia, or isolated hemihyperplasia) are usually screened with ultrasonography every 3 months until they reach at least age 8 years.[ 78 ][ 122 ] Early-stage, asymptomatic, small Wilms tumors may be discovered and potentially removed with renal-sparing surgery.[ 122 ]
Tumor screening programs for each overgrowth syndrome have been suggested. These programs were based on published age, incidence of tumor type, and recommendations from the 2016 American Association for Cancer Research (AACR) Childhood Cancer Predisposition Workshop. Although data about different cancer risks based on genetic or epigenetic subgroups for certain syndromes are emerging, and subgroup-specific recommendations have been developed in Europe, these practices have not been adopted in the United States. The AACR workshop committee proposed a uniform screening approach for all syndromes associated with a greater-than-1% risk of Wilms tumor. Additional screening for hepatoblastoma by serum alpha-fetoprotein (AFP) measurement and ultrasonography is also recommended for patients with Beckwith-Wiedemann syndrome, trisomy 18, and Simpson-Golabi-Behmel syndrome.[ 123 ]
-
Beckwith-Wiedemann syndrome. Approximately 8% of patients with Beckwith-Wiedemann syndrome will develop a malignancy, with the most common being either Wilms tumor or hepatoblastoma, although adrenal tumors can also occur.[
93
]
The AACR workshop committee has published screening guidelines that include Beckwith-Wiedemann syndrome.[ 123 ] Screening for hepatoblastoma or adrenal tumors with abdominal ultrasonography and serum AFP usually begins at birth or when the syndrome is diagnosed and continues until age 4 years. After age 4 years, most hepatoblastomas will have occurred, and imaging may be limited to renal ultrasonography, which is quicker and does not require fasting before the exam.[ 124 ] Screening for Wilms tumor usually continues until age 7 years. Physical examination by a specialist (geneticist or pediatric oncologist) is recommended twice per year, and ongoing education regarding tumor manifestations, reinforcing the rationale for screening and compliance with the screening regimen, is discussed.[ 123 ]
Proposed screening guidelines for Wilms tumor are available for patients with Beckwith-Wiedemann syndrome who have undergone molecular subtyping [ 93 ] (refer to the Genomics of Wilms Tumor section of this summary for more information about the molecular subtypes). The four main molecular subtypes of Beckwith-Wiedemann syndrome (ICR1-GoM, ICR2-LoM, UPD, and CDKN1C mutation) are characterized by specific genotype-phenotype correlations, including tumor risk.
Proposed screening for specific molecular subtypes of Beckwith-Wiedemann syndrome is as follows:
- Patients with a defect of the ICR1 region (ICR1-GoM) and UPD should undergo abdominal ultrasonography every 3 months until age 8 to 10 years; a clinical examination of the abdomen and muscle mass occurs monthly for the first year and then at 3-month intervals, between ultrasonography scans, until age 6 years.
- For patients with loss of imprinting at ICR2 (ICR2-LoM), an abdominal ultrasonography is performed at the time of clinical or molecular diagnosis; only patients with organomegaly or severe hemihyperplasia require surveillance by ultrasonography scans. Monthly clinical examinations are performed for the first 2 years, followed by clinical examinations every 3 to 6 months until age 6 years.
- Patients with a CDKN1C mutation are not at high risk of developing Wilms tumor. There are no data to support routine screening.
- On the basis of a literature search of patients with Beckwith-Wiedemann spectrum and Wilms tumor where the age at diagnosis was compared against data collected through the Surveillance, Epidemiology, and End Results (SEER) program, screening patients with Beckwith-Wiedemann spectrum seems to significantly decrease the age and stage at the time of diagnosis in this population. Screening until age 7 years is effective in detecting close to 95% of all Wilms tumors in Beckwith-Wiedemann spectrum. Screening until age 30 months may also prove useful for patients with IC2 LoM, consistent with the recommendations for hepatoblastoma screening in this population.[ 125 ]
- Hemihyperplasia. Children with hemihyperplasia are also at risk of developing liver tumors, adrenal tumors, and Wilms tumor (risk, 3%–4%). Screening with abdominal ultrasonography and serum AFP is suggested until age 4 years. After age 4 years, most hepatoblastomas will have occurred, and imaging may be limited to renal ultrasonography, which is quicker and does not require fasting before the exam.[ 123 ]
- Sporadic aniridia. Newborns born with sporadic aniridia should undergo molecular testing for deletion analysis of PAX6 and WT1. If a deletion of WT1 is observed, the child should be screened with ultrasonography every 3 months until age 8 years, and the parents should be educated about the need for early identification and treatment of Wilms tumor.[ 78 ][ 126 ][ 127 ]
- Children of survivors of bilateral Wilms tumor. Although the risk of Wilms tumor in the children of survivors of bilateral Wilms tumor is unknown and likely varies with the gene in which the mutation occurred, some experts recommend screening such children with serial ultrasonography examinations every 3 months until age 8 years.[ 66 ]
- Bohring-Opitz syndrome. Bohring-Opitz syndrome is a rare genetic condition associated with ASXL1 mutations. Screening with abdominal ultrasonography every 3 to 4 months in the first 8 years of life has been suggested because of the 7% incidence of a renal neoplastic process in patients with Bohring-Opitz syndrome.[ 40 ]
- Simpson-Golabi-Behmel syndrome. Affected males with Simpson-Golabi-Behmel syndrome with GPC3 mutations or deletions have an approximate 10% risk of Wilms tumor. Regular age-dependent screening for tumors, including abdominal ultrasonography, urinalysis, and biochemical markers, is recommended for males with Simpson-Golabi-Behmel syndrome, although the true benefit has not been determined. Carrier females are not at increased risk of Wilms tumor and do not require surveillance.[ 78 ]
- Klippel-Trénaunay syndrome. The risk of Wilms tumor in children with Klippel-Trénaunay syndrome (a unilateral limb overgrowth syndrome) was no different than the risk in the general population when assessed using the NWTS database. Routine ultrasonography surveillance is not recommended.[ 128 ]
- Perlman syndrome. Perlman syndrome is a rare congenital overgrowth syndrome that has an autosomal recessive inheritance pattern. A molecular diagnosis can be made by the presence of inactivating mutations in DIS3L2 on chromosome 2q37.1. Fifty-three percent of children will die in the neonatal period. The kidneys show nephroblastomatosis in about 75% of cases. It is recommended that patients who survive the neonatal period be offered regular surveillance similar to that offered to patients with Beckwith-Wiedemann syndrome.[ 123 ]
- DICER1 syndrome. Cystic nephroma is seen in 10% of families presenting with pleuropulmonary blastoma, typically occurring before age 4 years. Rare progression to anaplastic sarcoma of the kidney may occur. DICER1 syndrome includes an elevated risk of Wilms tumor, which is not a consequence of a prior cystic nephroma. Surveillance with abdominal ultrasonography, similar to Beckwith-Wiedemann syndrome, may be offered, but the age at which this should be stopped or reduced in frequency has not been established.[ 129 ][ 130 ] Thirteen years is the oldest reported age of a Wilms tumor diagnosis in a DICER1 mutation carrier.[ 106 ][ 131 ]
Genetic counseling
The frequency of malformations observed in patients with Wilms tumor underlines the need for genetic counseling, molecular and genetic explorations, and follow-up.
A French study concluded that patients need to be referred for genetic counseling if they have one of the following:[ 7 ]
- One major abnormality such as:
- Beckwith-Wiedemann symptoms (macroglossia, neonatal or postnatal macrosomia, abdominal wall defects, or visceromegaly); or
- One condition such as:
- Hemihyperplasia.
- Overgrowth syndrome.
- Mental retardation.
- Aniridia.
- Diffuse mesangial sclerosis.
- Two or more minor malformations such as:
- Inguinal or umbilical hernia.
- Hypospadias.
- Renal abnormalities.
- Ectopic testis.
Simple oncological follow-up is indicated when there is no malformation or when there is only one minor malformation.[ 7 ]
After genetic counseling takes place, testing for WT1 mutations should be considered for patients who have the following:
- Bilateral Wilms tumor.
- Familial Wilms tumor.
- Wilms tumor and age younger than 6 months.
- Genitourinary abnormality.
- Mental retardation association.
Testing for an 11p15 abnormality should be considered for patients exhibiting any symptoms of Beckwith-Wiedemann syndrome, hemihyperplasia, or bilateral or familial Wilms tumor.
Clinical Features of Wilms Tumor
Most Wilms tumor patients present asymptomatically with an abdominal mass noticed by a parent or pediatrician on a well-child visit. In children with known predisposing clinical syndromes, renal tumors can be found during routine screening. Other findings include the following:
- A lump, swelling, or pain in the abdomen. Most children present with an asymptomatic mass that is noted when they are bathed or dressed. Abdominal pain is present in 40% of children.
- Blood in the urine. Gross hematuria occurs in about 18% of children with Wilms tumor at presentation, and microscopic hematuria is seen in 24% of patients.[ 132 ]
- Hypertension. About 25% of children have hypertension at presentation, which is attributed to activation of the renin-angiotensin system.
- Hypercalcemia. Symptomatic hypercalcemia can sometimes be seen at presentation of rhabdoid tumors.
- Constitutional symptoms such as fever, anorexia, and weight loss occur in 10% of cases.
Children with Wilms tumor or other renal malignancies may also come to medical attention as a result of the following:
- Vascular obstruction or metastasis, including pulmonary symptoms caused by lung metastasis.
- Abdominal pain caused by liver metastasis, prominent abdominal wall vessels, or varicocele due to inferior vena cava obstruction.
- Pulmonary embolus (rare).
Diagnostic and Staging Evaluation for Wilms Tumor
Tests and procedures used to diagnose and stage Wilms tumor and other childhood kidney tumors include the following:
- Physical exam and history. Children with a renal mass are carefully assessed for signs of associated syndromes such as aniridia, developmental delay, hypospadias, cryptorchidism, pseudohermaphrodism, overgrowth, and hemihyperplasia.
- Complete blood count (CBC).
- Liver function test.
- Renal function test.
- Urinalysis.
- Abdominal imaging.
- Abdominal x-ray.
- Ultrasonography exam of the abdomen. Ultrasonography exam of the abdomen is often performed before a more definitive computed tomography (CT) scan with contrast or magnetic resonance imaging (MRI) with contrast of the abdomen is done. This procedure is unnecessary after the definitive diagnostic study has been performed.
- CT scan with contrast or MRI of abdomen.[
133
]
- CT scan of the abdomen will confirm the renal origin of the mass and determine whether there are bilateral tumors.[ 134 ] About 5% of renal masses thought to be Wilms tumor on the basis of clinical and radiological findings are diagnosed as another condition.[ 135 ]
- A review of children with bilateral Wilms tumor demonstrated that only 0.25% of bilateral tumors were missed with modern helical CT scans, all of which were small tumors.[ 136 ]
- Preoperative assessment by imaging of intravascular extension of Wilms tumor is essential to guide management. Four percent of Wilms tumor patients present with inferior vena cava or atrial involvement and 11% with renal vein involvement, which may lead to differences in management. Embolization of a caval thrombus to the pulmonary artery is rare but can be lethal, and the presence of a thrombus must be identified preoperatively to prevent this occurrence and guide treatment. A report from the COG shows that CT can accurately identify cavoatrial thrombus, obviating the need for ultrasonography if CT has already been performed.[ 137 ]
- Ascites beyond the cul-de-sac is most predictive of preoperative Wilms tumor rupture, regardless of attenuation. In the presence of ascites, fat stranding around the tumor and the presence of retroperitoneal fluid are highly predictive of rupture.[ 134 ]
- Chest x-ray is unnecessary if chest CT is performed initially.
- CT scan of chest. The common sites of metastases for Wilms tumor are the lung and liver. Approximately 15% of patients will present with pulmonary metastases. CT scanning provides the most sensitive method of detecting metastatic lung nodules.
- Fluorine F 18-fludeoxyglucose (18F-FDG) positron emission tomography (PET)-CT. Wilms tumor is 18F-FDG avid, and 18F-FDG PET-CT imaging adds clinically applicable information to conventional CT scan imaging. PET-CT may be particularly helpful in patients with bilateral disease or those receiving preoperative chemotherapy. 18F-FDG PET-CT highlights FDG-avid areas in the tumor and metastases, which corresponds to histologically confirmed active disease.[ 138 ]
- von Willebrand disease work-up. About 1% to 8% of patients presenting with Wilms tumor have an acquired form of von Willebrand disease, although many are asymptomatic. von Willebrand multimers bind to Wilms tumor, reducing the plasma concentration to low levels.[ 139 ] Some clinicians recommend evaluation for von Willebrand disease before surgery.
- Biopsy or resection and the issue of bilateral Wilms. In children with a renal mass that clinically appears to be resectable Wilms tumor, biopsy is not performed so that tumor cells are not spread during the biopsy. A biopsy would upstage such a patient to stage III. In North America, the initial treatment in most cases is primary nephrectomy. If a primary nephrectomy cannot be performed, a biopsy, either open or with multiple cores, is required. The contraindications to primary nephrectomy are the following:
- Extension of tumor thrombus to the level of the hepatic veins. These patients should be considered for tumor resection after neoadjuvant chemotherapy when there is evidence of regression of the vena caval thrombus regardless of the degree of response of the primary tumor.
- The tumor involves contiguous structures whereby the only means of removing the kidney tumor requires removal of the other structure (e.g., spleen, pancreas, colon but excluding the adrenal gland and diaphragm). While Wilms tumors are frequently adherent to adjacent organs, in most cases, there is not frank invasion by the tumor and the organs can be dissected freely from the tumor. Radical en bloc resection (e.g., partial hepatectomy) is not generally warranted. If removal of a small section of diaphragm, psoas muscle, or tip of the pancreas allows the tumor to be removed intact, this is considered safe and appropriate.
- The surgeon's judgment that nephrectomy would result in significant or unnecessary morbidity/mortality, significant tumor spill, or residual tumor.[ 140 ]
- If there is pulmonary compromise because of extensive pulmonary metastases or, in rare cases, hepatic disease.
Biopsy tissue from inoperable Wilms tumor obtained before chemotherapy may be used for histologic review and initial treatment decisions. However, the use of biopsy to determine histology in an inoperable tumor remains controversial because biopsy may cause local tumor spread and the histologic classification of the Wilms tumor cannot be determined by biopsy.[ 140 ]
If a child undergoes a biopsy as the first procedure, they are considered stage III because they have gross residual tumors.
In children with a renal mass that clinically appears to be stage I or stage II Wilms tumor, biopsy is not performed so that tumor cells are not spread during the biopsy. A biopsy would upstage such a patient to stage III. Nephrectomy (in North America) or chemotherapy (in Europe) is performed instead. Therefore, the diagnostic pathology is first seen when the nephrectomy specimen is examined.
Children who have bilateral Wilms tumor are often treated without a biopsy.[ 141 ]
Biopsy of a renal mass may be indicated if the mass is atypical by radiographic appearance for Wilms tumor, and the patient is not going to undergo immediate nephrectomy. Biopsy tissue from inoperable Wilms tumor obtained before chemotherapy may be used for histologic review and initial treatment decisions.[ 140 ]
Anaplastic histology can be difficult to detect in any biopsy sample because of tumor heterogeneity. It is important to recognize that data from NWTS-4 and NWTS-5 (COG-Q9401/NCT00002611) have shown that, because of the histologic heterogeneity of Wilms tumor, a significant number of patients have anaplastic histology that is missed during an upfront biopsy whether it be a core needle biopsy or an incisional biopsy [ 142 ] but revealed at the time of definitive surgery after chemotherapy.
Detection of a contralateral renal lesion in a child with Wilms tumor can change the stage and initial management of the patient, indicating a role for a renal-sparing approach without up-front surgery. The detection of contralateral renal lesions is important at baseline imaging because routine intraoperative exploration of the contralateral kidney is no longer recommended on the basis of the results of the NWTS-4 study.[ 133 ][ 136 ] If the initial imaging studies suggests a bilateral process, treatment as a bilateral Wilms tumor is recommended by the authors. If the origin of the other lesion is indeterminate, the authors recommend pathological assessment of that lesion before proceeding with a nephrectomy.[ 133 ][ 136 ]
In children with bilateral Wilms tumor, biopsy can be avoided if the child is of typical age and radiographic appearance. This was assessed on the COG AREN0534 (NCT00945009) study where 187 of 189 patients were treated initially without a biopsy. All had Wilms tumors. If after 6 weeks of therapy, response was less then 30% by RECIST1.1 criteria, bilateral biopsies were performed to assess for anaplasia, stromal differentiation, and rhabdomyomatous changes. If anaplasia was detected, the chemotherapy treatment was changed. If the other two were detected, further chemotherapy was unlikely to result in tumor shrinkage and definitive surgery was recommended by the authors.[ 141 ]
- Lymph node sampling is required to locally stage all Wilms tumor patients. Lymph nodes have shown to be of major prognostic value for both short-term and long-term survival. Gross inspection is notoriously inaccurate, with a false-negative rate of 31.3% and a false-positive rate of 18.1%.[ 143 ]
About 5% of renal masses thought to be Wilms tumor on the basis of clinical and radiological findings are diagnosed as another condition.[ 144 ]
For patients with suspected Wilms tumor, additional preoperative staging studies are performed to assess intravascular extension or rupture of Wilms tumor.[ 135 ]
- Intravascular extension of the Wilms tumor. Preoperative assessment of intravascular extension of Wilms tumor is essential to guide management. The presence of intravenous tumor thrombus in the lumen of the renal vein, inferior vena cava, and right atrium has been reported in up to 11.3% of Wilms tumor patients and may lead to differences in management.
In North America, local staging of Wilms tumor is performed with CT or MRI of the abdomen and pelvis. Contrast-enhanced CT for Wilms tumor patients has high sensitivity and specificity for detection of cavoatrial tumor thrombus that may impact surgical approach. Routine Doppler evaluation after CT has been performed but is not necessarily required.[ 137 ] If the tumor is at or above the hepatic veins, a biopsy with preoperative chemotherapy is suggested because of the lower rate of serious intraoperative complications. Before surgical approach to the renal mass is performed, large tumor thrombi need to be controlled, especially when they extend above the hepatic vein, to avoid embolization of the tumor. In some cases, cardiopulmonary bypass is required.[ 145 ]
- Wilms tumors can rupture before surgery. The term rupture is used to imply a break in the tumor capsule before surgery, whereas the term spill refers to a break in the tumor during surgery. Based on their similar diagnostic performances, either CT or MRI can be used to detect rupture. Although imaging findings of rupture have high specificity (88%), the diagnosis of rupture has to be confirmed at surgery. Imaging alone cannot be used for initial staging because of the low sensitivity and specificity for preoperative rupture and lymph node status.[ 146 ]
Prognosis and Prognostic Factors for Wilms Tumor
Wilms tumor is a curable disease in most affected children. Since the 1980s, the 5-year survival rate for Wilms tumor with favorable histology (FH) has been consistently above 90%.[ 147 ] This favorable outcome occurred despite reductions in the length of therapy, dose of radiation, extent of fields irradiated, and the percentage of patients receiving radiation therapy.[ 148 ]
The prognosis for patients with Wilms tumor depends on the following:[ 149 ][ 150 ][ 151 ][ 152 ]
- Histopathologic features of the tumor (FH vs. anaplastic histology). (Refer to the Histologic Findings in Wilms Tumor section of this summary for more information.)
- Stage of disease at diagnosis.
- Molecular features of the tumor such as 1q gain and loss of heterozygosity of 1p and 16q. 1q gain, affecting 28% of Wilms tumors, is the most powerful predictor of outcome and is associated with an adverse outcome.[ 97 ][ 98 ][ 99 ]
- Age. Older age is associated with adverse prognosis.[ 153 ]
Older adolescents and adults with Wilms tumor
Wilms tumor in patients older than 16 years is rare, with an incidence rate of less than 0.2 cases per 1 million per year.[ 154 ] In Europe, the median age at diagnosis for adult patients with Wilms tumor (defined as age >15 years) is 34 years; however, patients older than 60 years have been reported.[ 154 ] Three percent of Wilms tumors occur in adults. Wilms tumor represents less than 1% of all renal tumors in adults and may be an unexpected finding after nephrectomy for presumed renal cell carcinoma, which is the most common adult renal cancer.
The outcome for adolescent and young adult (AYA) patients (aged 15 to 39 years) is inferior to the outcome for children. In an analysis of patients with Wilms tumor in the Surveillance, Epidemiology, and End Results (SEER) database, AYA patients (n = 104) had a statistically worse 5-year OS (69% vs. 94%; P < .001) than did pediatric patients (n = 2,574).[ 155 ][Level of evidence: 3iA] Better results have been reported for adults when they are treated in pediatric trials. The National Wilms Tumor Study (NWTS) Group reported the outcomes for adult patients with Wilms tumor from the NWTS-1, -2, and -3 trials. The 3-year OS rate for adults on the NWTS-1 trial was 24% (compared with 74% in children) and improved to a 5-year OS rate of 82.6% on the NWTS-3 trial, although the number of adult patients treated on each trial was 31 or fewer.[ 156 ][ 157 ][ 158 ] These data suggest that many adults with Wilms tumor, if treated appropriately, can expect to be cured, especially if the tumor has not spread and/or is completely resected. The inferior outcome of the adult patients may be the result of differences in tumor biology between children and adults, incorrect diagnosis, inadequate staging (e.g., more likely to be staged as localized disease or to not receive lymph node sampling), undertreatment/poor compliance (e.g., not receiving radiation therapy), unfamiliarity of medical oncologists and pathologists with Wilms tumors in adults (possibly leading to diagnostic error and delay), delays in initiating the appropriate risk-adapted therapy, and lack of specific treatment protocols for adults. For adults with refractory or recurrent disease, screening for potential therapeutic targets in the tumor should be considered.[ 159 ]
The following recommendations from the renal tumor committees of the International Society of Pediatric Oncology (SIOP) and COG encourage a uniform approach to improve outcome for adults with Wilms tumor.[ 160 ]
- Consult with a pediatric oncologist who has experience with the treatment of Wilms tumor as soon as a histological diagnosis is suspected.
- Avoid delaying the start of chemotherapy. Ideally, chemotherapy, and radiation therapy if necessary, should be started by day 14 postnephrectomy, although delaying the start until day 30 is acceptable.
- Be alert for toxicity of vincristine (neurotoxicity) and dactinomycin (hepatic toxicity) in adults.
- Register patients in pediatric renal tumor trials if studies are available and the patients are eligible.
Histologic Findings in Wilms Tumor
Although most patients with a histologic diagnosis of Wilms tumor do well with current treatment, approximately 10% of patients have histopathologic features that are associated with a worse prognosis, and in some types, with a high incidence of relapse and death. Wilms tumor can be separated into the following two prognostic groups on the basis of tumor and kidney histopathology:
Favorable histology (FH)
Histologically, Wilms tumor mimics the triphasic development of a normal kidney consisting of blastemal, epithelial (tubules), and stromal cell types. Not all tumors are triphasic, and monophasic patterns may present diagnostic difficulties.
While associations between histologic features and prognosis or responsiveness to therapy have been suggested, with the exception of anaplasia, none of these features have reached statistical significance in North American treatment algorithms, and therefore, do not direct the initial therapy.[ 161 ]
Anaplastic histology
Anaplastic histology accounts for about 10% of Wilms tumor cases. Anaplastic histology is the single most important histologic predictor of response and survival in patients with Wilms tumor. Tumors occurring in older patients (aged 10–16 years) have a higher incidence of anaplastic histology.[ 162 ] In bilateral tumors, 12% to 14% have been reported to have anaplastic histology in one kidney.[ 163 ][ 164 ]
The following two histologic criteria must be present to confirm the diagnosis of anaplasia:
- Presence of multipolar polyploid mitotic figures with marked nuclear enlargement.
- Hyperchromasia.
Changes on 17p consistent with mutations in the TP53 gene have been associated with foci of anaplastic histology.[ 109 ] Focal anaplasia is defined as the presence of one or more sharply localized regions of anaplasia in a primary tumor. All of these factors lend support to the hypothesis that anaplasia evolves as a late event from a subpopulation of Wilms tumor cells that have acquired additional genomic lesions.[ 165 ] Focal anaplasia does not confer as poor a prognosis as does diffuse anaplasia.[ 151 ][ 166 ][ 167 ]
Anaplasia correlates best with responsiveness to therapy rather than to tumor aggressiveness. It is most consistently associated with poor prognosis when it is diffusely distributed and when identified at advanced stages. These tumors are more resistant to the chemotherapy traditionally used in children with FH Wilms tumor.[ 151 ]
Nephrogenic rests
Nephrogenic rests are abnormally retained embryonic kidney precursor cells arranged in clusters. Nephrogenic rests are found in about 1% of unselected pediatric autopsies, 35% of kidneys with unilateral Wilms tumor, and nearly 100% of kidneys with bilateral Wilms tumor.[ 168 ][ 169 ] Preoperative chemotherapy does not appear to affect the overall prevalence of nephrogenic rests. Congenital anomalies have been reported in 12% of patients with nephrogenic rests, including in 9% of patients with unilateral Wilms tumor and in 33% of patients with bilateral disease.[ 6 ]
The term nephroblastomatosis is defined as the presence of diffuse or multifocal nephrogenic rests. Nephrogenic rests can be subclassified according to the category of rest (intralobar or perilobar nephrogenic rests) and their growth phase (incipient or dormant nephrogenic rests, hyperplastic nephrogenic rests, and regressing or sclerosing nephrogenic rests). Diffuse hyperplastic perilobar nephroblastomatosis represents one unique category of nephroblastomatosis that forms a thick rind around one or both kidneys and is considered a preneoplastic condition. Distinguishing between Wilms tumor and diffuse hyperplastic perilobar nephrogenic rests may be a challenge, and it is critical to examine the juncture between the lesion and the surrounding renal parenchyma. Incisional biopsies are of no diagnostic value unless they include the margin between the lesion and the normal renal parenchyma.[ 170 ]
The type and percentage of nephrogenic rests vary in patients with unilateral or bilateral disease. Patients with bilateral Wilms tumor have a higher proportion of perilobar rests (52%) than of intralobar or combined rests (32%) and higher relative proportions of rests, compared with patients with unilateral tumors (18% perilobar and 20% intralobar or both).[ 81 ] Intralobar nephrogenic rests have been associated with stromal-type Wilms tumor and younger age at diagnosis.[ 6 ]
Patients with any type of nephrogenic rest in a kidney removed for nephroblastoma are considered at increased risk for tumor formation in the remaining kidney. This risk decreases with patient age.[ 47 ]
Bilateral diffuse hyperplastic perilobar nephroblastomatosis is generally treated with chemotherapy to reduce the risk of developing Wilms tumor; however, the risk of developing Wilms tumor remains high, 55% in one series.[ 170 ] Patients who have been treated with chemotherapy for a prolonged period of time remain at high risk of developing Wilms tumor. If these patients develop Wilms tumor, they have a poorer prognosis than do other bilateral Wilms tumor patients, presumably because of the increased incidence of anaplasia in these cases (more than one-third of cases), and perhaps as a result of the development and selection of anaplasia in the surviving abnormal kidney cells.[ 170 ][ 171 ]
Extrarenal nephrogenic rests are rare and may develop into extrarenal Wilms tumor.[ 172 ]
Stage Information for Wilms Tumor
Both the results of the imaging studies and the surgical and pathologic findings at nephrectomy are used to determine the stage of disease. The stage is the same for tumors with FH or anaplastic histology. Thus, the stage information is characterized by a statement of both criteria (for example, stage II, FH or stage II, anaplastic histology).[ 161 ][ 173 ]
The staging system was originally developed by the NWTS Group and is still used by the COG. The staging system used in North America and incidence by stage are outlined below.[ 161 ]
Stage I
In stage I Wilms tumor (43% of patients), all of the following criteria must be met:
- Tumor is limited to the kidney and is completely resected.
- The renal capsule is intact.
- The tumor is not ruptured or biopsied before being removed.
- No involvement of renal sinus vessels.
- No evidence of the tumor at or beyond the margins of resection.
- All lymph nodes sampled are negative.
For a tumor to qualify for certain therapeutic protocols such as very low-risk stage I, regional lymph nodes must be examined microscopically. Lymph node sampling is strongly recommended for all patients, even in the absence of clinical abnormal nodes, to achieve the most accurate stage.
Stage II
In stage II Wilms tumor (20% of patients), the tumor is completely resected, and there is no evidence of tumor at or beyond the margins of resection. The tumor extends beyond the kidney as evidenced by any one of the following criteria:
- There is regional extension of the tumor (i.e., penetration of the renal capsule, or extensive invasion of the soft tissue of the renal sinus, as discussed below).
- Blood vessels in the nephrectomy specimen outside the renal parenchyma, including those of the renal sinus, contain tumor cells. Margins are clear.
- Vascular extension of tumor is considered stage II only if it is completely removed en bloc in the nephrectomy specimen.
All lymph nodes sampled are negative.
Rupture or spillage confined to the flank, including biopsy of the tumor, is now included in stage III by the COG Renal Tumor Committee (COG RTC); however, data to support this approach are controversial.[ 140 ][ 174 ]
Stage III
In stage III Wilms tumor (21% of patients), there is postsurgical residual nonhematogenous tumor that is confined to the abdomen. Any one of the following may occur:
- Lymph nodes in the abdomen or pelvis are involved by tumor. (Lymph node involvement in the thorax or other extra-abdominal sites is a criterion for stage IV.)
- The tumor has penetrated through the peritoneal surface.
- Tumor implants are found on the peritoneal surface.
- Gross or microscopic tumor remains postoperatively (e.g., tumor cells are found at the margin of surgical resection on microscopic examination).
- The tumor is not completely resectable because of local infiltration into vital structures.
- Tumor rupture before surgery or any spill during surgery is considered stage III.
- Any biopsy is performed, regardless of type—Tru-cut biopsy, open biopsy, or fine-needle aspiration—before the tumor is removed.
- The tumor is removed in more than one piece (e.g., tumor cells are found in a separately excised adrenal gland; a tumor thrombus in the renal vein is removed separately from the nephrectomy specimen). Extension of the primary tumor in the vena cava into the thoracic vena cava and heart is considered stage III, rather than stage IV, even though outside the abdomen—and it can even be stage II if completely resected en bloc with the nephrectomy specimen.
Lymph node involvement and microscopic residual disease are reported as highly predictive of outcome in patients with stage III FH Wilms tumor.[ 175 ]
Stage IV
In stage IV Wilms tumor (11% of patients), one of the following is present:
- Hematogenous metastases (lung, liver, bone, brain).
- Lymph node metastases outside the abdominopelvic region.
The presence of tumor within the adrenal gland is not interpreted as metastasis and staging depends on all other staging parameters present. According to the criteria described above, the primary tumor is assigned a local stage, which determines local therapy. For example, a patient may have stage IV, local stage III disease.
Stage V
In stage V Wilms tumor (5% of patients), bilateral involvement by tumor is present at diagnosis. The current paradigm treats all patients with bilateral Wilms tumor the same for the first 6 or 12 weeks. After definitive surgery, the treatment is based on the highest stage of the remaining kidneys and the posttreatment pathology.[ 141 ]
Treatment of Wilms Tumor
Treatment option overview for Wilms tumor
Because of the relative rarity of Wilms tumor, all patients with this tumor should be considered for entry into a clinical trial. Treatment planning by a multidisciplinary team of cancer specialists (pediatric surgeon and/or pediatric urologist, pediatric radiation oncologist, and pediatric oncologist) who have experience treating children with Wilms tumor is necessary to determine and implement optimal treatment.
Most randomized clinical studies for treatment of children with Wilms tumor have been conducted by two large clinical groups (COG RTC and SIOP). Differences between the two groups affect staging and classification. There are two standard approaches to Wilms tumor treatment: the COG RTC uses immediate surgery for all unilateral tumors and the SIOP uses preoperative chemotherapy as the first step in treatment. Both groups use postoperative chemotherapy, except for selected cases who do not receive chemotherapy, and in advanced stages, radiation therapy is used in a risk-adapted approach.
- COG RTC (includes the previous NWTS Group): The NWTS Group established standard treatment for Wilms tumor in North America, consisting of initial nephrectomy (when feasible) followed by chemotherapy and, in some patients, radiation therapy.[ 176 ][ 177 ][ 178 ] This approach allows for early and accurate histologic diagnosis, collection of biologic materials unaltered by therapy, and staging information, such as the presence of tumor spill or tumor involvement in lymph nodes, before chemotherapy is administered.
- SIOP: SIOP is a European consortium whose trials provide preoperative chemotherapy before definitive resection for patients with renal tumors. This results in fewer tumor spills during surgery and lower postoperative stage.[ 179 ] When the histological features of Wilms tumors from patients who underwent immediate surgery were compared with the histological features of those who received preoperative chemotherapy, preoperative chemotherapy was shown to significantly alter the histology, with fewer blastemal and mixed histology types in the tumors. Additionally, there were fewer stage III tumors in the preoperative chemotherapy group.[ 180 ]
- Both SIOP and COG treat infants younger than 6 months with a primary nephrectomy.[ 181 ]
This summary focuses on the NWTS (now COG RTC) results and studies.
The major treatment and study conclusions of NWTS-1, NWTS-2, NWTS-3, NWTS-4, and NWTS-5 are as follows:
- Routine, postoperative radiation therapy of the flank is not necessary for children with stage I tumors or stage II tumors with FH when postnephrectomy combination chemotherapy consisting of vincristine and dactinomycin is administered.[ 178 ]
- The prognosis for patients with stage III FH is best when treatment includes either (a) dactinomycin, vincristine, doxorubicin, and 10.8 Gy of radiation therapy to the flank; or (b) dactinomycin, vincristine, and 20 Gy of radiation therapy to the flank. Whole abdominal radiation is indicated for extensive intraperitoneal disease or widespread intraperitoneal tumor spill with possible boost to gross residual disease.[ 178 ]
- The addition of cyclophosphamide at the protocol dose (10 mg/kg/d for 3 days every 6 weeks) to the combination of vincristine, dactinomycin, and doxorubicin does not improve prognosis for patients with stage IV FH tumors.[ 178 ]
- A single dose of dactinomycin per course (stages I–II FH, stage I anaplastic histology, stage III FH, stages III–IV, or stages I–IV clear cell sarcoma of the kidney) is equivalent to the divided-dose courses, results in the same EFS, achieves greater dose intensity, and is associated with less toxicity and expense.[ 182 ]
- Eighteen weeks of therapy is adequate for patients with stage I and stage II FH, and stage III and IV patients can be treated with 6 months of therapy instead of 15 months.[ 148 ][ 176 ][ 182 ][ 183 ][ 184 ]
- Gain of 1q is associated with inferior survival in unilateral FH Wilms tumor. It is the single most powerful predictor of outcome, and in the presence of 1q gain, neither 1p nor 16q loss is significant. In the absence of 1q gain in unilateral FH Wilms tumor, 1p and/or 16q loss retain some prognostic significance and are associated with a higher risk of recurrence.[ 97 ][ 99 ]
Surgery
The following operative principles have also evolved from NWTS trials:
- The most important role
for the surgeon is to ensure complete tumor removal without rupture and assess the extent of disease. Radical nephrectomy and lymph node
sampling via a transabdominal or thoracoabdominal incision is the procedure
of choice.[
185
] A flank incision is not performed because it provides limited exposure to the kidney.
For patients with resectable tumors, preoperative biopsy or intraoperative biopsy is not performed because either would upstage the tumor in the current COG staging system.[ 185 ]
- Routine exploration of the contralateral kidney is not necessary if technically adequate imaging studies do not suggest a bilateral process. If the initial imaging studies suggest bilateral kidney involvement, treatment approaches should facilitate renal-sparing surgery.[ 136 ]
- About 2% of Wilms tumor cases have ureteral involvement. The presence of gross hematuria, nonfunctioning kidney, or hydronephrosis suggests the tumor may extend into the ureter, and cystoscopy is recommended. En bloc resection to avoid tumor spill is recommended.[ 186 ]
- The surgeon needs to be aware of the risk of intraoperative spill, especially in patients who have right-sided and large tumors, as noted in a review of cases of intraoperative spill among 1,131 patients registered on COG study AREN03B2 (NCT00898365).[ 187 ]
- Even if stage IV disease (e.g., pulmonary metastases) is evident on imaging, resection of the renal tumor should be considered. Treatment of local stage I or II Wilms tumor in the setting of distant metastasis does not require local radiation therapy.
Renal-sparing surgery remains controversial and is not recommended, except for children with the following:[ 188 ][ 189 ]; [ 190 ][Level of evidence: 3iiB]
- A solitary kidney.
- Predisposition to bilateral tumors. Some children who are predisposed to bilateral tumors and who have very small tumors detected by screening ultrasonography may be considered for renal-sparing surgery to preserve renal tissue.[ 188 ]
- Horseshoe kidney. Wilms tumor arising in a horseshoe kidney is rare, and accurate preoperative diagnosis is important for planning the operative approach. Primary resection is possible in most cases. Inoperable cases can usually be resected after chemotherapy.[ 191 ]
- Wilms tumor in infants with Denys-Drash or Frasier syndrome (to delay the need for dialysis).
Renal-sparing surgery does not appear to be feasible for most patients at the time of diagnosis because of the location of the tumor within the kidney, even in patients with very low-risk tumors.[ 192 ] In North America, renal-sparing surgery (partial nephrectomy) of unilateral Wilms tumor after administration of chemotherapy to shrink the tumor mass is considered investigational.[ 193 ][ 194 ]
Hilar and periaortic lymph node sampling is appropriate even if the nodes appear normal.[ 185 ][ 195 ] Furthermore, any suspicious node basin is sampled. Margins of resection, residual tumor, and any suspicious node basins are marked with titanium clips.
Wilms tumor rarely invades adjacent organs; therefore, resection of contiguous organs is seldom indicated. There is an increased incidence of complications occurring in more extensive resections that involve removal of additional organs beyond the diaphragm and adrenal gland. This finding has led to the recommendation in current COG protocols that patients in whom nephrectomy will require removal of additional organs should be considered for initial biopsy, neoadjuvant chemotherapy, and then secondary resection.[ 196 ] Primary resection of liver metastasis is not recommended.[ 197 ]
Chemotherapy
Preoperative chemotherapy before nephrectomy is indicated in the following situations, which have been listed previously under situations requiring a biopsy (refer to the Diagnostic and Staging Evaluation for Wilms Tumor section of this summary for more information):[ 185 ][ 196 ][ 198 ][ 199 ][ 200 ][ 201 ]
- Wilms tumor in a solitary kidney.
- Synchronous bilateral Wilms tumor.
- Extension of tumor thrombus in the inferior vena cava above the level of the hepatic veins. About 4% of Wilms tumor patients present with inferior vena cava or atrial involvement, and 11% of patients present with renal vein involvement. Embolization of a caval thrombus to the pulmonary artery is rare but can be lethal, and the presence of a thrombus must be identified preoperatively to prevent this occurrence and guide treatment.[ 137 ][ 145 ]
- Tumor involves contiguous structures whereby the only means of removing the kidney tumor requires removal of the other structures (e.g., spleen, pancreas, or colon but excluding the adrenal gland).
- Inoperable Wilms tumor.
- Pulmonary compromise resulting from extensive pulmonary metastases.
Preoperative chemotherapy follows a biopsy. The biopsy may be performed through a flank approach.[ 145 ][ 202 ][ 203 ][ 204 ][ 205 ][ 206 ] Adequate tissue is essential for accurate histological assessment and molecular studies. Preoperative chemotherapy includes doxorubicin in addition to vincristine and dactinomycin unless anaplastic histology is present; in such cases, chemotherapy then includes treatment with regimen I (refer to Table 2). The chemotherapy generally makes tumor removal easier by decreasing the size and vascular supply of the tumor; it may also reduce the frequency of surgical complications.[ 140 ][ 145 ][ 196 ][ 198 ][ 207 ][ 208 ]
In North America, the use of preoperative chemotherapy in patients with evidence of a contained preoperative rupture has been suggested to avoid intraoperative spill, but this is controversial.[ 209 ][ 210 ] The preoperative diagnosis of a contained retroperitoneal rupture on CT is difficult, even for experienced pediatric radiologists.[ 134 ]
Newborns and all infants younger than 12 months who will be treated with chemotherapy require a 50% reduction in chemotherapy dose compared with the dose given to older children.[ 211 ] Dosing for infants (younger than 12 months) will be calculated per kilogram of weight, not body surface area. This reduction diminishes the toxic effects reported in children in this age group enrolled in NWTS studies while maintaining an excellent overall outcome.[ 212 ]
Liver function tests in children with Wilms tumor are monitored closely during the early course of therapy because hepatic toxic effects (sinusoidal obstructive syndrome, previously called veno-occlusive disease) have been reported in these patients.[ 213 ][ 214 ] Dactinomycin or doxorubicin should not be administered during radiation therapy. Patients who develop renal failure while undergoing therapy can continue receiving chemotherapy with vincristine, dactinomycin, and doxorubicin. Vincristine and doxorubicin can be given at full doses; however, dactinomycin is associated with severe neutropenia. Reductions in dosing these agents may not be necessary, but accurate pharmacologic and pharmacokinetic studies are needed while the patient is receiving therapy.[ 215 ][ 216 ]
Augmentation of therapy improves EFS for patients with FH Wilms tumor and loss of heterozygosity of 1p/16q. In the AREN0532 (NCT00352534) and AREN0533 (NCT00379340) trials, patients with stage I and stage II FH Wilms tumor who were treated with the DD-4A regimen (dactinomycin, vincristine, and doxorubicin) demonstrated a 4-year EFS rate of 87.3%, compared with the 4-year EFS rate of 68.8% (P = .042) for stage I and stage II patients treated on the NWTS-5 trial. Patients with stage III and stage IV disease had a 4-year EFS rate of 90.2% when treated with regimen M, compared with a 61.3% 4-year EFS rate (P = .001) for stage III and stage IV patients treated on the NWTS-5 trial. Trends toward improved 4-year survival rates were seen in stage I and II patients and in stage III and IV patients.[ 217 ][Level of evidence: 3iiiDi]
Postoperative radiation therapy to the tumor bed is required when a biopsy is performed or in the setting of local tumor stage III. In a study of 1,488 patients with Wilms tumors who underwent surgery and radiation therapy, delay in starting radiation therapy after surgery of greater than 14 days was associated with an increased risk of mortality for patients with nonmetastatic Wilms tumor.[ 218 ][Level of evidence: 3iiiA]
Table 2 describes the accepted chemotherapy regimens used to treat Wilms tumor.
Table 2. Accepted Chemotherapy Regimens for Wilms Tumor Regimen Name Regimen Description Regimen EE-4A [ ] Vincristine, dactinomycin × 18 weeks postnephrectomy Regimen DD-4A [ ] Vincristine, dactinomycin, doxorubicin × 24 weeks; baseline nephrectomy or biopsy with subsequent nephrectomy Regimen I [ ] Vincristine, doxorubicin, cyclophosphamide, etoposide × 24 weeks postnephrectomy Regimen M [ ] Vincristine, dactinomycin, doxorubicin, cyclophosphamide, and etoposide with subsequent radiation therapy Regimen UH1 [ ] Vincristine, doxorubicin, cyclophosphamide, carboplatin, and etoposide × 30 weeks + radiation therapy Regimen UH2 [ ] Vincristine, doxorubicin, cyclophosphamide, carboplatin, etoposide, vincristine, and irinotecan × 36 weeks + radiation therapy Radiation therapy
Radiation therapy is used to improve local control and treat sites of metastatic disease. Radiation therapy has historically been dependent on stage and histology, but more recently is also guided by the tumor molecular signature.[ 221 ]
-
COG approach:
Upfront surgery provides histologic confirmation and tumor extent, providing the rationale for adjuvant therapy, including radiation therapy. Besides histology, postoperative risk factors for worse local control include: (1) incomplete resection, (2) positive margins, and (3) nodal involvement. Radiation therapy is not used in patients with stage I or stage II FH Wilms tumor. For patients with FH Wilms tumor, flank or abdominal radiation therapy is used for treatment in stage III tumors. In cases of unfavorable histology (focal or diffuse anaplasia), flank or abdominal radiation therapy is indicated for all patients. (Refer to Table 3 for more information.)
- Flank radiation therapy covers the tumor bed, involved nodal region, and entire adjacent vertebral bodies at 10.8 Gy in 1.8-Gy fractions. The dose of radiation therapy is based on the results of the NWTS-3 study in which there was no increase in abdominal relapse for stage III FH patients receiving 10 Gy versus 20 Gy with DD-4A chemotherapy.[ 222 ]
- Whole-abdominal radiation therapy is 10.5 Gy in 1.5-Gy fractions and is used to treat diffuse spill or peritoneal metastasis.
- In the closed COG AREN0321 (NCT00335556) study, the radiation therapy dose to the tumor bed was 10.8 Gy in 1.8-Gy fractions, with the exception of patients with stage III diffuse anaplasia, where a dose of 19.8 Gy in 1.8-Gy fractions was used. This remains the current standard of treatment.
- Results of the early NWTS studies (1 and 2) suggested that a radiation therapy delay of more than 10 days from surgery results in worse local control, particularly in unfavorable histology Wilms tumor.[ 223 ][ 224 ] However, no difference in local control was found if radiation therapy was delayed more than 10 days from surgery for patients with stages II to IV FH tumors treated on NWTS-3 or NWTS-4.[ 93 ] More recent data from the National Cancer Database confirms improved survival in patients with nonmetastatic Wilms tumor who received adjuvant radiation therapy less than or equal to 14 days postoperatively.[ 218 ]
- Results from the NWTS-3 and NWTS-4 trials indicate that there is no survival benefit of whole-lung irradiation in the setting of lung metastases seen on CT scan only.[ 225 ] Current COG guidelines allow for whole-lung irradiation omission in cases of FH disease without extrapulmonary metastases, loss of heterozygosity at 1p and 16q, and complete response at 6 weeks after vincristine, dactinomycin, and doxorubicin.[ 221 ] When whole-lung irradiation is given, a dose of 12 Gy in 1.5-Gy fractions is indicated for children older than 12 months and 10.5 Gy in 1.5-Gy fractions for patients younger than 12 months with pulmonary metastasis.
- Other sites of metastatic disease in Wilms tumor are uncommon and may include liver, extra-abdominal nodes, brain, and bone. In the COG AREN0533 (NCT00379340) study, the radiation therapy dose recommendations for patients younger than 16 years are 19.8 Gy in 1.8-Gy fractions to liver and gross residual nodes, 21.6 Gy in 1.8-Gy fractions to the whole brain with a boost of 10.8 Gy in 1.8-Gy fractions to gross metastatic disease in the brain, and 25.2 Gy in 14 fractions for bone metastasis. For patients older than 16 years, the radiation therapy dose to the whole brain and bone is increased to 30.6 Gy in 1.8-Gy fractions.
Table 3. Radiation Therapy Guidelines in Children’s Oncology Group AREN0532, AREN0533, and AREN0321 Protocols Local/Locoregional Disease XRT = radiation therapy. aRequires whole-abdominal XRT in 1.5 Gy daily fractions. Patients with diffuse unresectable peritoneal implants receive 21 Gy. bWhole-lung irradiation is given in 1.5 Gy daily fractions. cNot all patients receive radiation therapy. dA boost is given for macroscopic disease. Stage I Stage II Stage III Stage III (diffuse spill, peritoneal metastasis, preoperative rupture)a Favorable histology No XRT No XRT 10.8 Gy 10.5 Gy Focal anaplasia 10.8 Gy 10.8 Gy 10.8 Gy 10.5 Gy Diffuse anaplasia 10.8 Gy 10.8 Gy 19.8 Gy 10.5 Gy + 9 Gy boost Metastatic Disease Stage IV Lung Stage IV Liver Stage IV Brain Stage IV Bone Favorable histology 10.5 Gy for age <12 monthsb,c; 12 Gy for age >12 monthsb,c 19.8 Gy +/- 5.4 to 10.8 Gy boostd 21.6 Gy + 10.8 Gy boost for age <16 years; 30.6 Gy for age >16 years 25.2 Gy for age <16 years; 30.6 Gy for age >16 years Focal or diffuse anaplasia 10.5 Gy for age <12 monthsb; 12 Gy for age >12 monthsb 19.8 Gy +/- 5.4 to 10.8 Gy boostd 21.6 Gy + 10.8 Gy boost for age <16 years; 30.6 Gy for age >16 years 25.2 Gy for age <16 years; 30.6 Gy for age >16 years -
SIOP approach:
Children who need radiation therapy undergo postoperative treatment to the flank and/or metastatic sites on the basis of the experience of previous SIOP trials. The SIOP 1 to 9 trials demonstrated that preoperative radiation therapy or preoperative chemotherapy decreased the proportion of patients who developed tumor spillage, from more than 20% to 5%. The noninferiority of preoperative chemotherapy to preoperative radiation therapy in the SIOP 5 trial, and the concern over secondary malignancies with preoperative radiation therapy, led SIOP to recommend preoperative chemotherapy as the standard initial treatment.[ 179 ] Over time, the percentage of children who were treated with postoperative radiation therapy decreased, from more than 90% to 15% and 25% in SIOP trials 6 to 9, SIOP 93-01, and SIOP-2001, respectively.[ 177 ]
Treatment of stage I Wilms tumor
Table 4 provides an overview of the standard treatment options and survival data for patients with stage I Wilms tumor, based on published results.
Table 4. Overview of Standard Treatment Options for Stage I Wilms Tumora Histology 4-Year RFS or EFS 4-Year OS Treatment (refer to DA = diffuse anaplastic; EFS = event-free survival; FA = focal anaplastic; FH = favorable histology; LOH = loss of heterozygosity; OS = overall survival; RFS = relapse-free survival; XRT = radiation therapy. aSource: Grundy et al.,[ 99 ] Shamberger et al.,[ 152 ] Fernandez et al.,[ 221 ] Dix et al.,[ 217 ] and Daw et al.[ 226 ] bOne patient with a pulmonary relapse 4.12 years after diagnosis. FH <24 mo/tumor weight <550g 90% 100% Surgery, including lymph node biopsy only FH >24 mo/tumor weight >550g 94% RFS 98% Nephrectomy + lymph node sampling followed by regimen EE-4A FH with LOH 1p/16q (n = 8) 100% EFS 100% Nephrectomy + lymph node sampling followed by regimen DD-4A FA 100% 100% (n = 8) Nephrectomy + lymph node sampling followed by regimen DD-4A and XRT DA 100%b 100% (n = 10) Nephrectomy + lymph node sampling followed by regimen DD-4A and XRT The COG validated the hypothesis that nephrectomy only is appropriate therapy for patients younger than 2 years at diagnosis with stage I FH Wilms tumor that weighed less than 550 g in the AREN0532 (NCT00352534) trial. The NWTS-5 trial investigated this approach for children younger than 2 years at diagnosis with stage I FH Wilms tumor that weighed less than 550 g.
Evidence (surgery only for children younger than 2 years at diagnosis with stage I FH tumor that weighed <550 g):
- The AREN0532 (NCT00352534) trial was designed to confirm the findings from NWTS-5 that adjuvant chemotherapy could be omitted for children younger than 2 years at diagnosis with stage I FH Wilms tumor that weighed less than 550 g. A total of 116 patients met the criteria for very low-risk Wilms tumor and were enrolled on the study.[
152
][
221
][
227
]
- Twelve patients relapsed.
- The estimated 4-year EFS rate was 89.7%, and the OS rate was 100%.
- 11p15 methylation status was associated with relapse (20% relapse with loss of heterozygosity, 25% relapse with loss of imprinting, and 3.3% relapse with retention of the normal imprinting [P = .011]).
- Risk of developing metachronous Wilms tumor is very low in patients with very low-risk Wilms tumor who lack evidence of an underlying syndrome.
- The COG reported the outcomes for patients of all ages with stage I FH Wilms tumors showing epithelial-predominant histology. Approximately 20% of stage I FH Wilms tumors registered on AREN03B2 were epithelial predominant. In this group of 177 patients with stage I epithelial-predominant FH Wilms tumors, 117 patients were treated with EE4A, and 57 patients were classified as having a very low-risk Wilms tumor and were treated with observation only.[
228
][Level of evidence: 3iiiA]
- The 4-year EFS rate was 96.2%, and the OS rate was 100%.
- There was no statistical difference in EFS and OS based on age at diagnosis (<48 months and >48 months) or treatment (EE4A vs. observation only).
- There were six events. Three patients developed contralateral tumors after their initial diagnosis, and two of these patients had received adjuvant chemotherapy for their initial tumors. Three patients developed metastatic disease, and all of these patients had previously received EE4A as their primary therapy.
- The AREN0321 (NCT00335556) study demonstrated that outcomes for patients with stage I anaplastic Wilms tumor were improved with the addition of doxorubicin and flank radiation therapy to vincristine/dactinomycin therapy.[
226
]
- Four-year EFS and OS rate estimates were 100% in AREN0321, compared with 70% and 81.5%, respectively, in an updated analysis of 27 patients from NWTS-5 (median follow up, 13.3 years). One patient with diffuse anaplasia relapsed 4.12 years after diagnosis on the AREN0321 trial.
- The addition of doxorubicin and radiation therapy to AREN0321 was on the basis of the pattern of relapse observed in stage I anaplastic Wilms tumor in the abdomen and distant sites in the NWTS-5 trial.
- Retrospective analysis of all patients with stage I anaplastic Wilms tumor treated on NWTS-1 through NWTS-5 and AREN0321 showed a significant improvement in EFS for patients treated with doxorubicin (4-year EFS rate, 97.2% vs. 77.5%; P = .01), but no difference in EFS according to flank radiation therapy was shown (4-year EFS rate, 91.7% vs. 80.2%; P = .15).
- The rate of local recurrence was low (3.6%) and appeared to be similar for patients who received flank radiation therapy (4%) and patients who did not receive flank radiation therapy (6.2%). Local relapse occurred only in patients with diffuse anaplasia.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Treatment of stage II Wilms tumor
Table 5 provides an overview of the standard treatment options and survival data for patients with stage II Wilms tumor, based on published results.
Table 5. Overview of Standard Treatment Options for Stage II Wilms Tumora Histology 4-Year RFS or EFS 4-Year OS Treatment (refer to DA = diffuse anaplastic; EFS = event-free survival; FA = focal anaplastic; FH = favorable histology; LOH = loss of heterozygosity; OS = overall survival; RFS = relapse-free survival; XRT = radiation therapy. aSource: Grundy et al.,[ 99 ] Dome et al.,[ 151 ] Dix et al.,[ 217 ] and Daw et al.[ 220 ] FH 86% RFS 98% Nephrectomy + lymph node sampling followed by regimen EE-4A FH LOH 1p/16q (n = 24) 83% EFS 100% Nephrectomy + lymph node sampling followed by regimen DD-4A FA 80% EFS 80% (n = 5) Nephrectomy + lymph node sampling followed by abdominal XRT and regimen DD-4A DA 84% EFS 84% (n = 19) Nephrectomy + lymph node sampling followed by abdominal XRT and regimen UH1 On NWTS-3, NWTS-4, and NWTS-5, patients with intraoperative spill were divided into two groups: (1) those with diffuse spillage involving the whole abdominal cavity; and (2) those with local spillage confined to the flank. Patients with diffuse spillage were treated with radiation therapy to the entire abdomen and three-drug chemotherapy (vincristine, dactinomycin, and doxorubicin), whereas patients with local spillage were treated with vincristine and dactinomycin only. On the basis of an analysis of patients treated on NWTS-3 and NWTS-4 indicating that patients with stage II disease and local spillage had inferior OS compared with patients with stage II disease without local spillage, COG studies treat patients with local spillage with doxorubicin and flank radiation.[ 229 ] This approach is controversial and has not been tested; therefore, it should not be considered standard.
In a review of 499 patients from NWTS-4 with stage II FH Wilms tumor, 95 of the patients experienced tumor spill. The 8-year RFS and OS rates for patients who experienced intraoperative tumor spill and were treated with vincristine and dactinomycin without flank radiation therapy were lower, at 75.7% and 90.3%, than the 85% and 95.6% rates for those who did not experience tumor spill. None of these differences achieved statistical significance.[ 174 ]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Treatment of stage III Wilms tumor
Table 6 provides an overview of the standard treatment options and survival data for patients with stage III Wilms tumor, based on published results.
Table 6. Overview of Standard Treatment Options for Stage III Wilms Tumora Histology 4-Year RFS or EFS 4-Year OS Treatment (refer to DA = diffuse anaplastic; EFS = event-free survival; FA = focal anaplastic; FH = favorable histology; LOH = loss of heterozygosity; OS = overall survival; RFS = relapse-free survival; XRT = radiation therapy. aSource: Grundy et al.,[ 99 ] Dome et al.,[ 151 ] Fernandez et al.,[ 230 ] Dix et al.,[ 217 ] and Daw et al.[ 220 ] FH (all patients) 88% EFS 97% Nephrectomy + lymph node sampling followed by abdominal XRT and regimen DD-4A FH (without LOH of 1p and/or 16q) and positive lymph nodes 85% EFS 97% Nephrectomy + lymph node sampling followed by abdominal XRT and regimen DD-4A FH (without LOH of 1p and/or 16q) and negative lymph nodes 97% EFS 99% Nephrectomy + lymph node sampling followed by abdominal XRT and regimen DD-4A FH (with LOH of 1p and 16q) (n = 31) 87% EFS 94% Nephrectomy + lymph node sampling followed by abdominal XRT and regimen M FA 88% RFS 100% (n = 8) Nephrectomy + lymph node sampling followed by abdominal XRT and regimen DD-4A FA (preoperative treatment) 71% RFS 71% (n = 7) Preoperative treatment with regimen DD-4A followed by nephrectomy + lymph node sampling and abdominal XRT DA 46% EFS 53% (n = 16) Preoperative treatment with regimen I followed by nephrectomy + lymph node sampling and abdominal XRT DA 82% EFS 91% (n = 23) Immediate nephrectomy + lymph node sampling followed by abdominal XRT and regimen UH1 Loss of heterozygosity of 1p or 16q was shown to influence EFS but not OS in 588 patients with stage III FH Wilms tumor treated on the COG AREN0532 protocol. When combined, lymph node status and loss of heterozygosity status provided a strong predictor of excellent EFS and OS when both were absent, with a 4-year EFS rate of 97%, and an OS rate of 99%.[ 230 ][Level of evidence: 2Di] The outcome was poorer for patients having both positive lymph nodes and loss of heterozygosity of 1p or 16q, with a 4-year EFS rate of 74%. However, the 4-year OS rate was not influenced, at 92%.[ 230 ] On the basis of these results, therapy was augmented for patients with loss of heterozygosity of 1p/16q for patients enrolled on the AREN0533 trial. Patients with stage III and stage IV Wilms tumor with loss of heterozygosity were treated with regimen M. The 4-year EFS rate was 90.2%, and the OS rate was 96.1%, compared with a 4-year EFS rate of 61.3% (P = .001) and a 4-year OS rate of 86.0% (P = .087) for patients in the NWTS-5 trial. There was a suggestion of improvement in survival; however, the study was not powered to detect differences in survival.[ 217 ][Level of evidence: 3iiiDi]
Early initiation of radiation therapy is a critical component of multimodal therapy for patients with nonmetastatic Wilms tumor. In a review of 1,488 patients with Wilms tumor who underwent surgery and radiation therapy, a surgery-to-radiation therapy interval of greater than 14 days was associated with an increased risk of mortality (hazard ratio, 2.13; P = .013). This underscores the importance of initiating radiation therapy within 14 days of surgery, which is specified in Wilms tumor treatment protocols.[ 218 ][Level of evidence: 3iiiA]
For patients classified as stage III purely on the basis of local spill, refer to the Treatment of stage II Wilms tumor section of this summary.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Treatment of stage IV Wilms tumor
Table 7 provides an overview of the standard treatment options and survival data for patients with stage IV Wilms tumor, based on published results.
Table 7. Overview of Standard Treatment Options for Stage IV Wilms Tumora Histology 4-Year RFS or EFS 4-Year OS Treatment (refer to CR = complete response; DA = diffuse anaplasia; EFS = event-free survival; FA = focal anaplasia; FH = favorable histology; LOH = loss of heterozygosity; OS = overall survival; RFS = relapse-free survival; XRT = radiation therapy. aSource: Grundy et al.,[ 99 ] Dome et al.,[ 151 ] Dix et al.,[ 219 ] Dix et al.,[ 217 ] and Daw et al.[ 220 ] bAbdominal XRT is planned according to local stage of renal tumor. cPulmonary XRT is reserved for patients with chest x-ray/chest computed tomography evidence of pulmonary metastases. dRefer to the AREN0533 (NCT00379340) study for more information. FH 76% RFS 86% Nephrectomy + lymph node sampling, followed by abdominal XRT,b radiation to sites of metastases, bilateral pulmonary XRT,c and regimen DD-4A FH (with isolated lung nodules) 85% EFS 96% Nephrectomy + lymph node sampling, followed by abdominal XRT,b +/- bilateral pulmonary XRT,c and regimen DD-4A or regimen Md FH (with isolated lung nodules with CR to DD-4A) 83% EFS 94% Nephrectomy + lymph node sampling, followed by abdominal XRTb and regimen DD-4A FH (with isolated lung nodules with incomplete response to DD-4A) 92% EFS 96% Nephrectomy + lymph node sampling, followed by abdominal XRTb and bilateral pulmonary XRTc and regimen M FH (with LOH of 1p and/or 16q) (n = 20) 95% EFS 100% Nephrectomy + lymph node sampling, abdominal XRTb radiation to sites of metastasesb, and regimen M FA 61% EFS 72% (n = 11) Nephrectomy + lymph node sampling, followed by abdominal XRT,b radiation to sites of metastases, bilateral pulmonary XRT,c and regimen DD-4A DA 33% EFS 33% (n = 10) Immediate nephrectomy + lymph node sampling followed by abdominal XRT,b radiation to sites of metastases, whole-lung XRT,c and regimen I DA (preoperative treatment) 60% EFS 70% (n = 10) Preoperative treatment with regimen UH2 followed by nephrectomy + lymph node sampling, followed by abdominal XRT,b radiation to sites of metastases, and whole-lung XRTc Stage IV disease is defined by the presence of hematogenous metastases to the lung, liver, bone, brain, or other sites, with the lung being the most common site. Historically, chest x-rays were used to detect pulmonary metastases. The introduction of CT created controversy because many patients had lung nodules detected by chest CT scans that were not seen on chest x-rays. Management of newly diagnosed patients with FH Wilms tumor who have lung nodules detected only by CT scans (with negative chest x-ray) has elicited controversy as to whether they need to be treated with additional intensive treatment that is accompanied by acute and late toxicities.
Evidence (treatment of pulmonary nodules detected by chest CT scan only):
- A retrospective review of 186 patients from NWTS-4 and NWTS-5 with CT-only–detected lung nodules reported on the use of doxorubicin, vincristine, and dactinomycin versus the use of two drugs.[
231
]
- Patients who received doxorubicin, vincristine, and dactinomycin with or without lung irradiation had a 5-year EFS rate of 80% versus an EFS rate of 56% for patients receiving only two drugs (P = .004).
- There was no difference in EFS according to whether the lung was irradiated.
- There was no difference in the 5-year OS rate (87% vs. 86%).
Retrospective studies from Europe have examined the impact of omitting pulmonary radiation in patients with pulmonary metastases diagnosed by chest x-ray. European investigators omitted radiation from the treatment of most patients with Wilms tumor and pulmonary metastases as identified on chest x-ray who were treated on the SIOP-93-01 (NCT00003804) trial. The European approach to renal tumors differs from the approach used in North America. All patients who were shown to have a renal tumor by imaging underwent 9 weeks of prenephrectomy chemotherapy consisting of vincristine, dactinomycin, and doxorubicin.
Evidence (omission of pulmonary irradiation):
- In a retrospective SIOP study, 234 newly diagnosed patients with Wilms tumor presenting with pulmonary metastases were treated according to the response of the pulmonary metastases to the prenephrectomy chemotherapy.[
232
]
- Patients who were in complete remission (67%) after 6 weeks of therapy continued with the same chemotherapy and did not require radiation to their lungs.
- The 5-year EFS rate was 77%, and the OS rate was 88%.
- Patients who had residual pulmonary metastases were evaluated for metastasectomy.
- Thirty-seven patients (17%) obtained complete remission with surgery, and their outcome was similar to that of the group of patients who were treated with chemotherapy. Tumor viability in the resected pulmonary metastases was not a factor for omitting radiation therapy.
- The 5-year EFS rate was 84%, and the OS rate was 92%.
- Patients with residual pulmonary metastases that were incompletely resected or inoperable received more aggressive chemotherapy consisting of ifosfamide/anthracycline alternating with carboplatin/etoposide for 9 weeks.
- Patients showing a complete remission at that time were spared pulmonary radiation and continued with chemotherapy, whereas patients with residual pulmonary metastases continued with additional chemotherapy (to complete 34 weeks) and pulmonary irradiation. The 5-year OS rate was 48%, compared with the OS rates for patients who responded to chemotherapy alone (88%) and those who underwent metastasectomy (92%) (P < .001).
- Patients with high-risk histologies, such as anaplastic Wilms tumor, were treated with more aggressive chemotherapy but had a poorer outcome, compared with that of patients with nonanaplastic histologies (5-year OS rate, 87% vs. 33%; P < .001).
- Patients who were in complete remission (67%) after 6 weeks of therapy continued with the same chemotherapy and did not require radiation to their lungs.
- The COG AREN0533 (NCT00379340) study applied a new strategy for patients with FH Wilms tumor and isolated lung metastases to improve EFS while reducing exposure to lung irradiation on the basis of the European experience. Therapy was adjusted on the basis of lung nodule response and tumor-specific loss of heterozygosity at 1p and 16q.[
219
][Level of evidence: 3iiiDi]
- Of the 292 patients enrolled in the study, 133 patients (42%) showed a complete lung nodule response after 6 weeks of DD-4A (vincristine, dactinomycin, doxorubicin) and continued receiving the same chemotherapy without lung radiation therapy. The 4-year EFS rate was 80%, and OS rate was 96%.
- Patients who had an incomplete lung nodule response (n = 145) or loss of heterozygosity at 1p/16q (n = 18) received lung radiation therapy and four cycles of cyclophosphamide/etoposide in addition to the DD-4A drugs (regimen M). The 4-year EFS rate was 89%, and the OS rate was 95% for the incomplete lung nodule response group without loss of heterozygosity. Of the patients with pulmonary metastases only and loss of heterozygosity, the 4-year EFS and OS rates were 100%.
- In a post hoc analysis of 1q gain in 212 patients enrolled in AREN0533 who had DNA available, patients with lung nodule complete remission with 1q gain had a significantly worse 4-year EFS rate (86% vs. 57%, P = .001) and trend toward inferior OS rates (97% vs. 89%). Relapses were predominantly pulmonary. There was no difference in outcome for patients with incomplete lung nodule response on the basis of 1q gain.
- Regimen M has a higher potential for late effects (increased risk of secondary leukemias and risk of infertility related to a cumulative dose of cyclophosphamide of 8.8 g/m2).
- COG showed that initial lung radiation therapy could be avoided in approximately 40% of patients. OS was excellent; however, there was a trend toward more events than expected (expected, 15% and observed, 20%; P = .052).
Although fewer patients were spared pulmonary radiation when treated in the COG trial than in the European trials, it is important to note several differences between the studies and why the studies cannot be directly compared.[ 219 ][ 232 ] Patients in Europe receive a more dose-dense regimen of dactinomycin and doxorubicin before their pulmonary metastases are reevaluated than do patients in North America (135 ug/kg dactinomycin and 100 mg/m2 doxorubicin in Europe, compared with 45 ug/kg dactinomycin and 45 mg/m2 of doxorubicin in North America). European studies allow lung radiation therapy to be omitted for patients with a complete remission achieved by chemotherapy or pulmonary metastasectomy, whereas radiation therapy was only omitted in the United States for patients with a complete remission with chemotherapy alone. Imaging studies were not centrally reviewed in the European studies, whereas they were in the United States, and the definition of complete remission may have been more stringent in the AREN0533 (NCT00379340) trial.
The presence of liver metastases at diagnosis is not an independent adverse prognostic factor in patients with stage IV Wilms tumor.[ 197 ]
In the AREN0321 (NCT00335556) study, the combination of vincristine and irinotecan (VI) was tested in an upfront window for patients with diffuse anaplastic Wilms tumor and measurable disease. Fourteen patients with stage IV diffuse anaplastic Wilms tumor with measurable disease received the window therapy; one patient achieved a complete response (CR), ten patients achieved partial responses (PRs), and no patients had stable disease. This resulted in a CR and PR rate of 79%. Patients who responded to VI in the window therapy had VI incorporated into their regimen (UH2). Because of the observed cardiac/pulmonary toxicities encountered in this trial, the study was interrupted and amended with reduced doses of doxorubicin, cyclophosphamide, and etoposide (when combined with carboplatin). Further study of the modified regimen is planned in patients with newly diagnosed diffuse anaplastic Wilms tumor.[ 220 ][Level of evidence: 3iiiDii]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
Treatment of stage V Wilms tumor and those predisposed to developing bilateral Wilms tumor
Currently, there is not a standard approach for the treatment of stage V Wilms tumor (bilateral Wilms tumor at diagnosis) and those predisposed to developing Wilms tumor; however, for the first time, a prospective study of the treatment of patients with bilateral Wilms tumor has been completed and provides guidance for the approach.[ 141 ]
Management of a child with bilateral Wilms tumor is very challenging. The goals of therapy are to eradicate all tumor and to preserve as much normal renal tissue as possible, with the hope of decreasing the risk of chronic renal failure among these children.[ 233 ]
Historically, based on the NWTS-4 and NWTS-5 trials and trials performed in Europe, patients with bilateral Wilms tumor have had a lower EFS and OS than have patients with localized Wilms tumor. The NWTS-4 study reported that the 8-year EFS rate for patients with bilateral FH Wilms tumor was 74%, and the OS rate was 89%; for patients with anaplastic histology, the EFS rate was 40%, and the OS rate was 45%.[ 164 ] The NWTS-5 study reported that the 4-year EFS rate for all bilateral Wilms tumor patients was 56%, and the OS rate was 81%; the 4-year EFS rates for patients with FH (65%), focal anaplastic histology (76%), and diffuse anaplastic histology (25%) were also reported.[ 99 ][ 151 ] Similar outcomes for patients with bilateral Wilms tumor have been reported in Europe.[ 163 ][ 234 ] In a single-institution experience in the Netherlands (N = 41), there was significant morbidity in terms of renal failure (32%) and secondary tumors (20%).[ 234 ] The incidence of end-stage renal failure in the Dutch study may be a reflection of a longer follow-up period.
Treatment options for stage V Wilms tumor include the following:
Preoperative chemotherapy and resection for bilateral Wilms tumor
For patients with bilateral Wilms tumor, the goal of therapy is to preserve as much renal tissue as possible without compromising overall outcome. This approach is used to avoid the late effect of end-stage renal disease, which can be caused by underlying germline genetic aberrations and treatment-related loss of functional renal tissue. End-stage renal disease occurs more frequently in patients with bilateral Wilms tumor (12% nonsyndromic) than in patients with unilateral Wilms tumor (<1%). Functional renal outcome is considerably better after bilateral nephron-sparing surgery than after other types of surgery.[ 141 ]
Traditionally, patients have undergone bilateral renal biopsies, with staging of each kidney followed by preoperative chemotherapy. In the first prospective multi-institutional treatment trial (COG AREN0534 [NCT00945009]), pretreatment biopsies were not required if results of imaging tests were consistent with Wilms tumor.[ 141 ] This approach was taken because the bilateral occurrence of non-Wilms renal tumors is very low. Also, core-needle and wedge biopsies are not highly successful in identifying anaplasia in Wilms tumor.[ 142 ] In the setting of an unusual clinical situation, such as age older than 10 years or atypical imaging features, when a diagnosis other than Wilms should be considered, a tissue diagnosis is obtained.[ 141 ]
For patients who are treated with preoperative chemotherapy, the tumor pathology needs to be evaluated after 4 to 8 weeks. For patients not treated in a clinical trial, the ideal time to perform a biopsy or resection is unknown because minimal shrinkage may reflect chemotherapy-induced differentiation or anaplastic histology. A planned attempt at resection or biopsy of apparently unresectable tumor is undertaken no later than 12 weeks from diagnosis. Continuing therapy without evaluating tumor pathology in a patient with bilateral Wilms tumor may miss anaplastic histology or chemotherapy-induced differentiation (including rhabdomyomatous differentiation) and thus increase toxicity for the patient without providing additional benefit for tumor control. Anaplastic histology occurs in 10% of patients with bilateral Wilms tumor, and these tumors respond poorly to chemotherapy.[ 164 ]
Once the diagnosis is confirmed, a complete resection is performed. Histologic confirmation of the diagnosis is not straightforward. In a series of 27 patients from NWTS-4, discordant pathology (unilateral anaplastic tumor) was seen in 20 cases (74%), which highlights the need to obtain tissue from both kidneys. Seven children who were later diagnosed with diffuse anaplastic tumors had core biopsies performed to establish the diagnosis; however, anaplasia was not found. Anaplasia was identified in only three of the nine patients when an open-wedge biopsy was performed and in seven of nine patients who had a partial or complete nephrectomy.[ 164 ]
The decision to administer chemotherapy and/or radiation therapy after biopsy or a second-look operation is dependent on the tumor's response to initial therapy. More aggressive therapy is required for patients with inadequate response to initial therapy observed at the second procedure or in the setting of anaplasia.[ 173 ][ 235 ][ 236 ]
End-stage renal disease is the most clinically significant morbidity in patients with bilateral Wilms tumor and can be caused by underlying germline genetic aberrations, as well as treatment-related loss of functional renal tissue. Long-term monitoring of renal function is required after treatment for bilateral disease.
Evidence (preoperative chemotherapy and resection for bilateral Wilms tumor):
- The first prospective study in bilateral Wilms tumor (AREN0534 [NCT00945009]) aimed to improve EFS and OS while preserving renal tissue by intensifying preoperative chemotherapy (utilizing three drugs—vincristine, dactinomycin, and doxorubicin), completing definitive surgery by 12 weeks from diagnosis, and modifying postoperative chemotherapy on the basis of histologic response.[
141
]
- For the arm that treated children with bilateral Wilms tumor, results showed that central review of imaging, surgical resection within 12 weeks of diagnosis, and response-based and histology-based postoperative therapy improved EFS and OS, when compared with the historical outcomes of children with bilateral Wilms tumor.
- For the 189 patients with bilateral Wilms tumor, 4-year EFS rate was 82.1% (95% confidence interval [CI], 73.5%–90.8%), and the OS rate was 94.9% (95% CI, 90.1%–99.7%). Because biopsy was not performed before treatment in this series, some of the patients enrolled may have had only nephrogenic rests and not a true Wilms tumor. This finding may have improved these survival figures over historical controls.
- One of the aims of the study was that 75% of patients undergo definitive surgery by 12 weeks. After induction chemotherapy, 163 of 189 patients (84%) underwent definitive surgical treatment in at least one kidney by 12 weeks, and 39% of patients retained parts of both kidneys.
- Chemotherapy after surgery was tailored according to histologic response. The 4-year EFS rate was 84.1% for FH tumors, 58.2% for anaplastic histology tumors, and 82% for blastemal-type tumors.
- Because of the higher risk of renal failure in patients with bilateral Wilms tumor than in patients with unilateral Wilms tumor, one of the goals of the study was that 50% of the patients undergo bilateral nephron-sparing surgery. This threshold was not met, with only 39% of patients successfully treated with bilateral nephron-sparing surgery.
- Based on the above study, the recommendation was to continue with three-drug preoperative chemotherapy for 6 to 12 weeks followed by nephron-sparing surgery whenever possible. After resection, postoperative therapy is based on the histology of the resected specimen. The disappointing use of nephron-sparing surgery in this study may have been because of the level of experience of the surgeons in this multi-institutional study.
- In a retrospective review of 93 children with bilateral Wilms tumor registered at Associazione Italiana di Ematologia e Oncologia Pediatrica (AIEOP) centers over a 21-year period, 43 patients were treated with vincristine and dactinomycin preoperatively and 37 patients were treated with vincristine, dactinomycin, and doxorubicin. The duration of preoperative chemotherapy ranged from 1 week to 40 weeks (median, 12 weeks).[
163
]
- The 4-year DFS rate was 67%, and the OS rate was 80%.
- There was a trend toward better EFS in nonmetastatic patients receiving vincristine, dactinomycin, and doxorubicin preoperatively (4-year EFS rate, 84%) than for patients receiving vincristine and dactinomycin (4-year EFS rate, 65%), but this was not significant.
- The bilateral renal parenchyma was preserved in 48% of patients.
- In a retrospective review of 49 patients with Wilms tumor who received preoperative therapy according to the SIOP-93-01 (NCT00003804) guidelines, the timing of surgery was determined when there was no longer imaging evidence of tumor regression. The mean treatment duration was 80 days before renal-sparing surgery.[
237
]
- The 5-year EFS rate was 83.4%, and the OS rate was 89.5%.
- All but one of the patients had renal-sparing surgery in at least one kidney.
- Despite the good survival, 14% of the patients developed end-stage renal disease.
- In a retrospective review from St. Jude Children's Research Hospital, investigators described their experience with preoperative chemotherapy followed by renal-sparing procedures in children with bilateral FH Wilms tumor.[
238
]
- In a series, 39 of 42 patients with bilateral FH Wilms tumor underwent successful bilateral renal-sparing procedures after receiving preoperative chemotherapy. Three patients underwent unilateral nephrectomy with contralateral nephron-sparing surgery. Three patients required early (within 4 months) repeat nephron-sparing surgery for residual tumor. In the long term, seven patients had local tumor recurrence, and three patients had intestinal obstruction.
- The OS rate was 86% (mean follow-up, 4.1 years). Of the six patients who died, five had diffuse anaplastic histology.
- All of the patients had an estimated glomerular filtration rate of more than 60 mL/min/1.73m2 at the last follow-up; none of the patients developed end-stage renal disease.
- The authors concluded that bilateral renal-sparing surgery is almost always feasible and can be done safely with good oncologic outcomes in patients with synchronous, bilateral Wilms tumor. It should be considered even if preoperative imaging studies suggest that the lesions are unresectable. Sparing of renal parenchyma is likely to help preserve renal function in children who are at significant risk of chronic renal insufficiency. Careful long-term follow-up is required to fully assess the potential progression of renal dysfunction.
- A follow-up review of these patients revealed the following: 8 of 36 patients underwent repeat nephron-sparing surgery, and an additional two patients required a third nephron-sparing surgery. Six of these patients were alive without disease at the 4.5-year follow-up. The two patients who died had blastemal-predominant histology.[ 239 ]
Renal transplant
Renal transplant for children with stage V Wilms tumor is usually delayed until 1 to 2 years have passed without evidence of malignancy because most relapses occur within 2 years of diagnosis.[ 240 ] Similarly, renal transplant for children with Denys-Drash syndrome and Wilms tumor, all of whom require bilateral nephrectomy, is generally delayed 1 to 2 years after completion of initial treatment.[ 240 ]
Treatment options under clinical evaluation
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
(Refer to the Treatment of Recurrent Childhood Kidney Tumors section of this summary for information about recurrent disease.)
Follow-up after treatment
For patients who have completed therapy for Wilms tumor and exhibit features consistent with genetic predisposition, such as bilateral Wilms tumor, screening involves renal ultrasonography examination every 3 months for metachronous tumors during the risk period for that particular syndrome (5 years for WT1-related syndromes; 8 years for Beckwith-Wiedemann syndrome).
Late effects after Wilms tumor therapy
Children treated for Wilms tumor are at increased risk of developing the following:
- Premature mortality after Wilms tumor diagnosis. In 1,441 5-year survivors of Wilms tumor, a substantial increase in cumulative mortality from 5.4% to 22.7% was noted from 30 to 50 years after Wilms tumor diagnosis. Excess deaths after 30 years were attributed to subsequent malignant neoplasms (50%) and cardiac-related causes (25%).[ 241 ] Radiation therapy was a risk factor for both outcomes.
- Subsequent malignant neoplasms.[ 241 ][ 242 ][ 243 ] Digestive cancers and breast cancer are the most frequent subsequent neoplasms, and radiation therapy is a risk factor. Women treated with lower doses of radiation to large volumes of breast tissue for a childhood cancer have a risk of breast cancer that is higher than previously recognized. The cumulative incidence of invasive breast cancer in Wilms tumor survivors who had received pulmonary radiation for metastatic Wilms tumor is nearly 15% by age 40 years.[ 244 ]
- Congestive heart failure. The risk of congestive heart failure is influenced by dose of doxorubicin received, radiation to the heart, and female sex.[ 243 ][ 245 ]
- Complications of pregnancy.[ 246 ]
- End-stage renal disease. The cumulative incidence of end-stage renal disease caused by chronic renal failure at 20 years from diagnosis of Wilms tumor is low, at 3.1% for patients with bilateral Wilms tumor and less than 1% for those with unilateral Wilms tumor.[ 81 ] Efforts, therefore, have been aimed toward reducing the intensity of therapy when possible.
(Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for a full discussion of the late effects of cancer treatment in children and adolescents.)
参考文献- Howlader N, Noone AM, Krapcho M, et al.: SEER Cancer Statistics Review (CSR) 1975-2016. Bethesda, Md: National Cancer Institute, 2019. Available online. Last accessed February 27, 2020.[PUBMED Abstract]
- Breslow N, Olshan A, Beckwith JB, et al.: Epidemiology of Wilms tumor. Med Pediatr Oncol 21 (3): 172-81, 1993.[PUBMED Abstract]
- Horner MJ, Ries LA, Krapcho M, et al.: SEER Cancer Statistics Review, 1975-2006. Bethesda, Md: National Cancer Institute, 2009. Also available online. Last accessed January 31, 2020.[PUBMED Abstract]
- Scott RH, Stiller CA, Walker L, et al.: Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet 43 (9): 705-15, 2006.[PUBMED Abstract]
- Narod SA, Hawkins MM, Robertson CM, et al.: Congenital anomalies and childhood cancer in Great Britain. Am J Hum Genet 60 (3): 474-85, 1997.[PUBMED Abstract]
- Vujanić GM, Apps JR, Moroz V, et al.: Nephrogenic rests in Wilms tumors treated with preoperative chemotherapy: The UK SIOP Wilms Tumor 2001 Trial experience. Pediatr Blood Cancer 64 (11): , 2017.[PUBMED Abstract]
- Dumoucel S, Gauthier-Villars M, Stoppa-Lyonnet D, et al.: Malformations, genetic abnormalities, and Wilms tumor. Pediatr Blood Cancer 61 (1): 140-4, 2014.[PUBMED Abstract]
- Gracia Bouthelier R, Lapunzina P: Follow-up and risk of tumors in overgrowth syndromes. J Pediatr Endocrinol Metab 18 (Suppl 1): 1227-35, 2005.[PUBMED Abstract]
- Lapunzina P: Risk of tumorigenesis in overgrowth syndromes: a comprehensive review. Am J Med Genet C Semin Med Genet 137 (1): 53-71, 2005.[PUBMED Abstract]
- Treger TD, Chowdhury T, Pritchard-Jones K, et al.: The genetic changes of Wilms tumour. Nat Rev Nephrol 15 (4): 240-251, 2019.[PUBMED Abstract]
- Clericuzio CL: Clinical phenotypes and Wilms tumor. Med Pediatr Oncol 21 (3): 182-7, 1993.[PUBMED Abstract]
- Fischbach BV, Trout KL, Lewis J, et al.: WAGR syndrome: a clinical review of 54 cases. Pediatrics 116 (4): 984-8, 2005.[PUBMED Abstract]
- Breslow NE, Norris R, Norkool PA, et al.: Characteristics and outcomes of children with the Wilms tumor-Aniridia syndrome: a report from the National Wilms Tumor Study Group. J Clin Oncol 21 (24): 4579-85, 2003.[PUBMED Abstract]
- Barbosa AS, Hadjiathanasiou CG, Theodoridis C, et al.: The same mutation affecting the splicing of WT1 gene is present on Frasier syndrome patients with or without Wilms' tumor. Hum Mutat 13 (2): 146-53, 1999.[PUBMED Abstract]
- Koziell AB, Grundy R, Barratt TM, et al.: Evidence for the genetic heterogeneity of nephropathic phenotypes associated with Denys-Drash and Frasier syndromes. Am J Hum Genet 64 (6): 1778-81, 1999.[PUBMED Abstract]
- Royer-Pokora B, Beier M, Henzler M, et al.: Twenty-four new cases of WT1 germline mutations and review of the literature: genotype/phenotype correlations for Wilms tumor development. Am J Med Genet A 127 (3): 249-57, 2004.[PUBMED Abstract]
- Pelletier J, Bruening W, Kashtan CE, et al.: Germline mutations in the Wilms' tumor suppressor gene are associated with abnormal urogenital development in Denys-Drash syndrome. Cell 67 (2): 437-47, 1991.[PUBMED Abstract]
- Mueller RF: The Denys-Drash syndrome. J Med Genet 31 (6): 471-7, 1994.[PUBMED Abstract]
- Barbaux S, Niaudet P, Gubler MC, et al.: Donor splice-site mutations in WT1 are responsible for Frasier syndrome. Nat Genet 17 (4): 467-70, 1997.[PUBMED Abstract]
- Porteus MH, Narkool P, Neuberg D, et al.: Characteristics and outcome of children with Beckwith-Wiedemann syndrome and Wilms' tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol 18 (10): 2026-31, 2000.[PUBMED Abstract]
- Rump P, Zeegers MP, van Essen AJ: Tumor risk in Beckwith-Wiedemann syndrome: A review and meta-analysis. Am J Med Genet A 136 (1): 95-104, 2005.[PUBMED Abstract]
- Choufani S, Shuman C, Weksberg R: Molecular findings in Beckwith-Wiedemann syndrome. Am J Med Genet C Semin Med Genet 163C (2): 131-40, 2013.[PUBMED Abstract]
- Eggermann T, Algar E, Lapunzina P, et al.: Clinical utility gene card for: Beckwith-Wiedemann Syndrome. Eur J Hum Genet 22 (3): , 2014.[PUBMED Abstract]
- Ibrahim A, Kirby G, Hardy C, et al.: Methylation analysis and diagnostics of Beckwith-Wiedemann syndrome in 1,000 subjects. Clin Epigenetics 6 (1): 11, 2014.[PUBMED Abstract]
- Brioude F, Lacoste A, Netchine I, et al.: Beckwith-Wiedemann syndrome: growth pattern and tumor risk according to molecular mechanism, and guidelines for tumor surveillance. Horm Res Paediatr 80 (6): 457-65, 2013.[PUBMED Abstract]
- Mussa A, Russo S, Larizza L, et al.: (Epi)genotype-phenotype correlations in Beckwith-Wiedemann syndrome: a paradigm for genomic medicine. Clin Genet 89 (4): 403-415, 2016.[PUBMED Abstract]
- Green DM, Breslow NE, Beckwith JB, et al.: Screening of children with hemihypertrophy, aniridia, and Beckwith-Wiedemann syndrome in patients with Wilms tumor: a report from the National Wilms Tumor Study. Med Pediatr Oncol 21 (3): 188-92, 1993.[PUBMED Abstract]
- DeBaun MR, Siegel MJ, Choyke PL: Nephromegaly in infancy and early childhood: a risk factor for Wilms tumor in Beckwith-Wiedemann syndrome. J Pediatr 132 (3 Pt 1): 401-4, 1998.[PUBMED Abstract]
- DeBaun MR, Tucker MA: Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. J Pediatr 132 (3 Pt 1): 398-400, 1998.[PUBMED Abstract]
- Milani D, Pezzani L, Tabano S, et al.: Beckwith-Wiedemann and IMAGe syndromes: two very different diseases caused by mutations on the same gene. Appl Clin Genet 7: 169-75, 2014.[PUBMED Abstract]
- Morris MR, Astuti D, Maher ER: Perlman syndrome: overgrowth, Wilms tumor predisposition and DIS3L2. Am J Med Genet C Semin Med Genet 163C (2): 106-13, 2013.[PUBMED Abstract]
- Astuti D, Morris MR, Cooper WN, et al.: Germline mutations in DIS3L2 cause the Perlman syndrome of overgrowth and Wilms tumor susceptibility. Nat Genet 44 (3): 277-84, 2012.[PUBMED Abstract]
- Golabi M, Leung A, Lopez C: Simpson-Golabi-Behmel Syndrome Type 1. In: Pagon RA, Adam MP, Bird TD, et al., eds.: GeneReviews. Seattle, Wash: University of Washington, 1993-2018, pp. Available online. Last accessed June 08, 2020.[PUBMED Abstract]
- Peterman CM, Fevurly RD, Alomari AI, et al.: Sonographic screening for Wilms tumor in children with CLOVES syndrome. Pediatr Blood Cancer 64 (12): , 2017.[PUBMED Abstract]
- Fagali C, Kok F, Nicola P, et al.: MLPA analysis in 30 Sotos syndrome patients revealed one total NSD1 deletion and two partial deletions not previously reported. Eur J Med Genet 52 (5): 333-6, 2009 Sep-Oct.[PUBMED Abstract]
- Isidor B, Bourdeaut F, Lafon D, et al.: Wilms' tumor in patients with 9q22.3 microdeletion syndrome suggests a role for PTCH1 in nephroblastomas. Eur J Hum Genet 21 (7): 784-7, 2013.[PUBMED Abstract]
- Cairney AE, Andrews M, Greenberg M, et al.: Wilms tumor in three patients with Bloom syndrome. J Pediatr 111 (3): 414-6, 1987.[PUBMED Abstract]
- Hartley AL, Birch JM, Tricker K, et al.: Wilms' tumor in the Li-Fraumeni cancer family syndrome. Cancer Genet Cytogenet 67 (2): 133-5, 1993.[PUBMED Abstract]
- Bourdeaut F, Guiochon-Mantel A, Fabre M, et al.: Alagille syndrome and nephroblastoma: Unusual coincidence of two rare disorders. Pediatr Blood Cancer 50 (4): 908-11, 2008.[PUBMED Abstract]
- Russell B, Johnston JJ, Biesecker LG, et al.: Clinical management of patients with ASXL1 mutations and Bohring-Opitz syndrome, emphasizing the need for Wilms tumor surveillance. Am J Med Genet A 167A (9): 2122-31, 2015.[PUBMED Abstract]
- Bonaïti-Pellié C, Chompret A, Tournade MF, et al.: Genetics and epidemiology of Wilms' tumor: the French Wilms' tumor study. Med Pediatr Oncol 20 (4): 284-91, 1992.[PUBMED Abstract]
- Winther JF, Sankila R, Boice JD, et al.: Cancer in siblings of children with cancer in the Nordic countries: a population-based cohort study. Lancet 358 (9283): 711-7, 2001.[PUBMED Abstract]
- Breslow NE, Olson J, Moksness J, et al.: Familial Wilms' tumor: a descriptive study. Med Pediatr Oncol 27 (5): 398-403, 1996.[PUBMED Abstract]
- Li FP, Williams WR, Gimbrere K, et al.: Heritable fraction of unilateral Wilms tumor. Pediatrics 81 (1): 147-9, 1988.[PUBMED Abstract]
- Ruteshouser EC, Huff V: Familial Wilms tumor. Am J Med Genet C Semin Med Genet 129 (1): 29-34, 2004.[PUBMED Abstract]
- Paulino AC, Thakkar B, Henderson WG: Metachronous bilateral Wilms' tumor: the importance of time interval to the development of a second tumor. Cancer 82 (2): 415-20, 1998.[PUBMED Abstract]
- Coppes MJ, Arnold M, Beckwith JB, et al.: Factors affecting the risk of contralateral Wilms tumor development: a report from the National Wilms Tumor Study Group. Cancer 85 (7): 1616-25, 1999.[PUBMED Abstract]
- Grundy P, Koufos A, Morgan K, et al.: Familial predisposition to Wilms' tumour does not map to the short arm of chromosome 11. Nature 336 (6197): 374-6, 1988.[PUBMED Abstract]
- Little SE, Hanks SP, King-Underwood L, et al.: Frequency and heritability of WT1 mutations in nonsyndromic Wilms' tumor patients: a UK Children's Cancer Study Group Study. J Clin Oncol 22 (20): 4140-6, 2004.[PUBMED Abstract]
- Hanks S, Perdeaux ER, Seal S, et al.: Germline mutations in the PAF1 complex gene CTR9 predispose to Wilms tumour. Nat Commun 5: 4398, 2014.[PUBMED Abstract]
- Scott RH, Douglas J, Baskcomb L, et al.: Constitutional 11p15 abnormalities, including heritable imprinting center mutations, cause nonsyndromic Wilms tumor. Nat Genet 40 (11): 1329-34, 2008.[PUBMED Abstract]
- Grønskov K, Olsen JH, Sand A, et al.: Population-based risk estimates of Wilms tumor in sporadic aniridia. A comprehensive mutation screening procedure of PAX6 identifies 80% of mutations in aniridia. Hum Genet 109 (1): 11-8, 2001.[PUBMED Abstract]
- Clericuzio C, Hingorani M, Crolla JA, et al.: Clinical utility gene card for: WAGR syndrome. Eur J Hum Genet 19 (4): , 2011.[PUBMED Abstract]
- Hoyme HE, Seaver LH, Jones KL, et al.: Isolated hemihyperplasia (hemihypertrophy): report of a prospective multicenter study of the incidence of neoplasia and review. Am J Med Genet 79 (4): 274-8, 1998.[PUBMED Abstract]
- Shanske AL: Trisomy 18 in a second 20-year-old woman. Am J Med Genet A 140 (9): 966-7, 2006.[PUBMED Abstract]
- Reid S, Renwick A, Seal S, et al.: Biallelic BRCA2 mutations are associated with multiple malignancies in childhood including familial Wilms tumour. J Med Genet 42 (2): 147-51, 2005.[PUBMED Abstract]
- Hirsch B, Shimamura A, Moreau L, et al.: Association of biallelic BRCA2/FANCD1 mutations with spontaneous chromosomal instability and solid tumors of childhood. Blood 103 (7): 2554-9, 2004.[PUBMED Abstract]
- Reid S, Schindler D, Hanenberg H, et al.: Biallelic mutations in PALB2 cause Fanconi anemia subtype FA-N and predispose to childhood cancer. Nat Genet 39 (2): 162-4, 2007.[PUBMED Abstract]
- Gadd S, Huff V, Walz AL, et al.: A Children's Oncology Group and TARGET initiative exploring the genetic landscape of Wilms tumor. Nat Genet 49 (10): 1487-1494, 2017.[PUBMED Abstract]
- Wegert J, Wittmann S, Leuschner I, et al.: WTX inactivation is a frequent, but late event in Wilms tumors without apparent clinical impact. Genes Chromosomes Cancer 48 (12): 1102-11, 2009.[PUBMED Abstract]
- Ruteshouser EC, Robinson SM, Huff V: Wilms tumor genetics: mutations in WT1, WTX, and CTNNB1 account for only about one-third of tumors. Genes Chromosomes Cancer 47 (6): 461-70, 2008.[PUBMED Abstract]
- Walz AL, Ooms A, Gadd S, et al.: Recurrent DGCR8, DROSHA, and SIX homeodomain mutations in favorable histology Wilms tumors. Cancer Cell 27 (2): 286-97, 2015.[PUBMED Abstract]
- Wegert J, Ishaque N, Vardapour R, et al.: Mutations in the SIX1/2 pathway and the DROSHA/DGCR8 miRNA microprocessor complex underlie high-risk blastemal type Wilms tumors. Cancer Cell 27 (2): 298-311, 2015.[PUBMED Abstract]
- Rakheja D, Chen KS, Liu Y, et al.: Somatic mutations in DROSHA and DICER1 impair microRNA biogenesis through distinct mechanisms in Wilms tumours. Nat Commun 2: 4802, 2014.[PUBMED Abstract]
- Torrezan GT, Ferreira EN, Nakahata AM, et al.: Recurrent somatic mutation in DROSHA induces microRNA profile changes in Wilms tumour. Nat Commun 5: 4039, 2014.[PUBMED Abstract]
- Dome JS, Huff V: Wilms Tumor Predisposition. In: Pagon RA, Adam MP, Bird TD, et al., eds.: GeneReviews. Seattle, Wash: University of Washington, 1993-2018, pp. Available online. Last accessed June 08, 2020.[PUBMED Abstract]
- Mahamdallie SS, Hanks S, Karlin KL, et al.: Mutations in the transcriptional repressor REST predispose to Wilms tumor. Nat Genet 47 (12): 1471-4, 2015.[PUBMED Abstract]
- Huff V: Wilms tumor genetics. Am J Med Genet 79 (4): 260-7, 1998.[PUBMED Abstract]
- Scott RH, Murray A, Baskcomb L, et al.: Stratification of Wilms tumor by genetic and epigenetic analysis. Oncotarget 3 (3): 327-35, 2012.[PUBMED Abstract]
- Corbin M, de Reyniès A, Rickman DS, et al.: WNT/beta-catenin pathway activation in Wilms tumors: a unifying mechanism with multiple entries? Genes Chromosomes Cancer 48 (9): 816-27, 2009.[PUBMED Abstract]
- Maiti S, Alam R, Amos CI, et al.: Frequent association of beta-catenin and WT1 mutations in Wilms tumors. Cancer Res 60 (22): 6288-92, 2000.[PUBMED Abstract]
- Gadd S, Huff V, Huang CC, et al.: Clinically relevant subsets identified by gene expression patterns support a revised ontogenic model of Wilms tumor: a Children's Oncology Group Study. Neoplasia 14 (8): 742-56, 2012.[PUBMED Abstract]
- Breslow NE, Beckwith JB, Perlman EJ, et al.: Age distributions, birth weights, nephrogenic rests, and heterogeneity in the pathogenesis of Wilms tumor. Pediatr Blood Cancer 47 (3): 260-7, 2006.[PUBMED Abstract]
- Fukuzawa R, Heathcott RW, More HE, et al.: Sequential WT1 and CTNNB1 mutations and alterations of beta-catenin localisation in intralobar nephrogenic rests and associated Wilms tumours: two case studies. J Clin Pathol 60 (9): 1013-6, 2007.[PUBMED Abstract]
- Perlman EJ, Gadd S, Arold ST, et al.: MLLT1 YEATS domain mutations in clinically distinctive Favourable Histology Wilms tumours. Nat Commun 6: 10013, 2015.[PUBMED Abstract]
- Diller L, Ghahremani M, Morgan J, et al.: Constitutional WT1 mutations in Wilms' tumor patients. J Clin Oncol 16 (11): 3634-40, 1998.[PUBMED Abstract]
- Perlman EJ, Grundy PE, Anderson JR, et al.: WT1 mutation and 11P15 loss of heterozygosity predict relapse in very low-risk wilms tumors treated with surgery alone: a children's oncology group study. J Clin Oncol 29 (6): 698-703, 2011.[PUBMED Abstract]
- Scott RH, Walker L, Olsen ØE, et al.: Surveillance for Wilms tumour in at-risk children: pragmatic recommendations for best practice. Arch Dis Child 91 (12): 995-9, 2006.[PUBMED Abstract]
- Lipska BS, Ranchin B, Iatropoulos P, et al.: Genotype-phenotype associations in WT1 glomerulopathy. Kidney Int 85 (5): 1169-78, 2014.[PUBMED Abstract]
- Lehnhardt A, Karnatz C, Ahlenstiel-Grunow T, et al.: Clinical and molecular characterization of patients with heterozygous mutations in wilms tumor suppressor gene 1. Clin J Am Soc Nephrol 10 (5): 825-31, 2015.[PUBMED Abstract]
- Lange J, Peterson SM, Takashima JR, et al.: Risk factors for end stage renal disease in non-WT1-syndromic Wilms tumor. J Urol 186 (2): 378-86, 2011.[PUBMED Abstract]
- Breslow NE, Takashima JR, Ritchey ML, et al.: Renal failure in the Denys-Drash and Wilms' tumor-aniridia syndromes. Cancer Res 60 (15): 4030-2, 2000.[PUBMED Abstract]
- Koesters R, Ridder R, Kopp-Schneider A, et al.: Mutational activation of the beta-catenin proto-oncogene is a common event in the development of Wilms' tumors. Cancer Res 59 (16): 3880-2, 1999.[PUBMED Abstract]
- Koesters R, Niggli F, von Knebel Doeberitz M, et al.: Nuclear accumulation of beta-catenin protein in Wilms' tumours. J Pathol 199 (1): 68-76, 2003.[PUBMED Abstract]
- Major MB, Camp ND, Berndt JD, et al.: Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science 316 (5827): 1043-6, 2007.[PUBMED Abstract]
- Rivera MN, Kim WJ, Wells J, et al.: An X chromosome gene, WTX, is commonly inactivated in Wilms tumor. Science 315 (5812): 642-5, 2007.[PUBMED Abstract]
- Fukuzawa R, Anaka MR, Weeks RJ, et al.: Canonical WNT signalling determines lineage specificity in Wilms tumour. Oncogene 28 (8): 1063-75, 2009.[PUBMED Abstract]
- Jenkins ZA, van Kogelenberg M, Morgan T, et al.: Germline mutations in WTX cause a sclerosing skeletal dysplasia but do not predispose to tumorigenesis. Nat Genet 41 (1): 95-100, 2009.[PUBMED Abstract]
- Grohmann A, Tanneberger K, Alzner A, et al.: AMER1 regulates the distribution of the tumor suppressor APC between microtubules and the plasma membrane. J Cell Sci 120 (Pt 21): 3738-47, 2007.[PUBMED Abstract]
- Satoh Y, Nakadate H, Nakagawachi T, et al.: Genetic and epigenetic alterations on the short arm of chromosome 11 are involved in a majority of sporadic Wilms' tumours. Br J Cancer 95 (4): 541-7, 2006.[PUBMED Abstract]
- Algar EM, St Heaps L, Darmanian A, et al.: Paternally inherited submicroscopic duplication at 11p15.5 implicates insulin-like growth factor II in overgrowth and Wilms' tumorigenesis. Cancer Res 67 (5): 2360-5, 2007.[PUBMED Abstract]
- Lennerz JK, Timmerman RJ, Grange DK, et al.: Addition of H19 'loss of methylation testing' for Beckwith-Wiedemann syndrome (BWS) increases the diagnostic yield. J Mol Diagn 12 (5): 576-88, 2010.[PUBMED Abstract]
- Mussa A, Molinatto C, Baldassarre G, et al.: Cancer Risk in Beckwith-Wiedemann Syndrome: A Systematic Review and Meta-Analysis Outlining a Novel (Epi)Genotype Specific Histotype Targeted Screening Protocol. J Pediatr 176: 142-149.e1, 2016.[PUBMED Abstract]
- Bliek J, Gicquel C, Maas S, et al.: Epigenotyping as a tool for the prediction of tumor risk and tumor type in patients with Beckwith-Wiedemann syndrome (BWS). J Pediatr 145 (6): 796-9, 2004.[PUBMED Abstract]
- Bjornsson HT, Brown LJ, Fallin MD, et al.: Epigenetic specificity of loss of imprinting of the IGF2 gene in Wilms tumors. J Natl Cancer Inst 99 (16): 1270-3, 2007.[PUBMED Abstract]
- Fukuzawa R, Breslow NE, Morison IM, et al.: Epigenetic differences between Wilms' tumours in white and east-Asian children. Lancet 363 (9407): 446-51, 2004.[PUBMED Abstract]
- Gratias EJ, Dome JS, Jennings LJ, et al.: Association of Chromosome 1q Gain With Inferior Survival in Favorable-Histology Wilms Tumor: A Report From the Children's Oncology Group. J Clin Oncol 34 (26): 3189-94, 2016.[PUBMED Abstract]
- Chagtai T, Zill C, Dainese L, et al.: Gain of 1q As a Prognostic Biomarker in Wilms Tumors (WTs) Treated With Preoperative Chemotherapy in the International Society of Paediatric Oncology (SIOP) WT 2001 Trial: A SIOP Renal Tumours Biology Consortium Study. J Clin Oncol 34 (26): 3195-203, 2016.[PUBMED Abstract]
- Grundy PE, Breslow NE, Li S, et al.: Loss of heterozygosity for chromosomes 1p and 16q is an adverse prognostic factor in favorable-histology Wilms tumor: a report from the National Wilms Tumor Study Group. J Clin Oncol 23 (29): 7312-21, 2005.[PUBMED Abstract]
- Messahel B, Williams R, Ridolfi A, et al.: Allele loss at 16q defines poorer prognosis Wilms tumour irrespective of treatment approach in the UKW1-3 clinical trials: a Children's Cancer and Leukaemia Group (CCLG) Study. Eur J Cancer 45 (5): 819-26, 2009.[PUBMED Abstract]
- Spreafico F, Gamba B, Mariani L, et al.: Loss of heterozygosity analysis at different chromosome regions in Wilms tumor confirms 1p allelic loss as a marker of worse prognosis: a study from the Italian Association of Pediatric Hematology and Oncology. J Urol 189 (1): 260-6, 2013.[PUBMED Abstract]
- Gratias EJ, Jennings LJ, Anderson JR, et al.: Gain of 1q is associated with inferior event-free and overall survival in patients with favorable histology Wilms tumor: a report from the Children's Oncology Group. Cancer 119 (21): 3887-94, 2013.[PUBMED Abstract]
- Hohenstein P, Pritchard-Jones K, Charlton J: The yin and yang of kidney development and Wilms' tumors. Genes Dev 29 (5): 467-82, 2015.[PUBMED Abstract]
- Foulkes WD, Priest JR, Duchaine TF: DICER1: mutations, microRNAs and mechanisms. Nat Rev Cancer 14 (10): 662-72, 2014.[PUBMED Abstract]
- Wu MK, Sabbaghian N, Xu B, et al.: Biallelic DICER1 mutations occur in Wilms tumours. J Pathol 230 (2): 154-64, 2013.[PUBMED Abstract]
- Palculict TB, Ruteshouser EC, Fan Y, et al.: Identification of germline DICER1 mutations and loss of heterozygosity in familial Wilms tumour. J Med Genet 53 (6): 385-8, 2016.[PUBMED Abstract]
- Chang HM, Triboulet R, Thornton JE, et al.: A role for the Perlman syndrome exonuclease Dis3l2 in the Lin28-let-7 pathway. Nature 497 (7448): 244-8, 2013.[PUBMED Abstract]
- Alessandri JL, Cuillier F, Ramful D, et al.: Perlman syndrome: report, prenatal findings and review. Am J Med Genet A 146A (19): 2532-7, 2008.[PUBMED Abstract]
- Bardeesy N, Falkoff D, Petruzzi MJ, et al.: Anaplastic Wilms' tumour, a subtype displaying poor prognosis, harbours p53 gene mutations. Nat Genet 7 (1): 91-7, 1994.[PUBMED Abstract]
- el Bahtimi R, Hazen-Martin DJ, Re GG, et al.: Immunophenotype, mRNA expression, and gene structure of p53 in Wilms' tumors. Mod Pathol 9 (3): 238-44, 1996.[PUBMED Abstract]
- Wallkamm V, Dörlich R, Rahm K, et al.: Live imaging of Xwnt5A-ROR2 complexes. PLoS One 9 (10): e109428, 2014.[PUBMED Abstract]
- Ooms AH, Gadd S, Gerhard DS, et al.: Significance of TP53 Mutation in Wilms Tumors with Diffuse Anaplasia: A Report from the Children's Oncology Group. Clin Cancer Res 22 (22): 5582-5591, 2016.[PUBMED Abstract]
- Williams RD, Al-Saadi R, Chagtai T, et al.: Subtype-specific FBXW7 mutation and MYCN copy number gain in Wilms' tumor. Clin Cancer Res 16 (7): 2036-45, 2010.[PUBMED Abstract]
- Muller E, Hudgins L: 9q22.3 Microdeletion. In: Pagon RA, Adam MP, Bird TD, et al., eds.: GeneReviews. Seattle, Wash: University of Washington, 1993-2018, pp. Available online. Last accessed June 08, 2020.[PUBMED Abstract]
- Garavelli L, Piemontese MR, Cavazza A, et al.: Multiple tumor types including leiomyoma and Wilms tumor in a patient with Gorlin syndrome due to 9q22.3 microdeletion encompassing the PTCH1 and FANC-C loci. Am J Med Genet A 161A (11): 2894-901, 2013.[PUBMED Abstract]
- Cajaiba MM, Bale AE, Alvarez-Franco M, et al.: Rhabdomyosarcoma, Wilms tumor, and deletion of the patched gene in Gorlin syndrome. Nat Clin Pract Oncol 3 (10): 575-80, 2006.[PUBMED Abstract]
- Williams RD, Chagtai T, Alcaide-German M, et al.: Multiple mechanisms of MYCN dysregulation in Wilms tumour. Oncotarget 6 (9): 7232-43, 2015.[PUBMED Abstract]
- Fievet A, Belaud-Rotureau MA, Dugay F, et al.: Involvement of germline DDX1-MYCN duplication in inherited nephroblastoma. Eur J Med Genet 56 (12): 643-7, 2013.[PUBMED Abstract]
- Martins AG, Pinto AT, Domingues R, et al.: Identification of a novel CTR9 germline mutation in a family with Wilms tumor. Eur J Med Genet 61 (5): 294-299, 2018.[PUBMED Abstract]
- Charlton J, Irtan S, Bergeron C, et al.: Bilateral Wilms tumour: a review of clinical and molecular features. Expert Rev Mol Med 19: e8, 2017.[PUBMED Abstract]
- Hu M, Fletcher J, McCahon E, et al.: Bilateral Wilms tumor and early presentation in pediatric patients is associated with the truncation of the Wilms tumor 1 protein. J Pediatr 163 (1): 224-9, 2013.[PUBMED Abstract]
- Murphy AJ, Davidoff AM: Bilateral Wilms Tumor: A Surgical Perspective. Children (Basel) 5 (10): , 2018.[PUBMED Abstract]
- Kalish JM, Doros L, Helman LJ, et al.: Surveillance Recommendations for Children with Overgrowth Syndromes and Predisposition to Wilms Tumors and Hepatoblastoma. Clin Cancer Res 23 (13): e115-e122, 2017.[PUBMED Abstract]
- Teplick A, Kowalski M, Biegel JA, et al.: Educational paper: screening in cancer predisposition syndromes: guidelines for the general pediatrician. Eur J Pediatr 170 (3): 285-94, 2011.[PUBMED Abstract]
- Mussa A, Duffy KA, Carli D, et al.: The effectiveness of Wilms tumor screening in Beckwith-Wiedemann spectrum. J Cancer Res Clin Oncol 145 (12): 3115-3123, 2019.[PUBMED Abstract]
- Hingorani M, Hanson I, van Heyningen V: Aniridia. Eur J Hum Genet 20 (10): 1011-7, 2012.[PUBMED Abstract]
- van Heyningen V, Hoovers JM, de Kraker J, et al.: Raised risk of Wilms tumour in patients with aniridia and submicroscopic WT1 deletion. J Med Genet 44 (12): 787-90, 2007.[PUBMED Abstract]
- Greene AK, Kieran M, Burrows PE, et al.: Wilms tumor screening is unnecessary in Klippel-Trenaunay syndrome. Pediatrics 113 (4): e326-9, 2004.[PUBMED Abstract]
- Schultz KAP, Rednam SP, Kamihara J, et al.: PTEN, DICER1, FH, and Their Associated Tumor Susceptibility Syndromes: Clinical Features, Genetics, and Surveillance Recommendations in Childhood. Clin Cancer Res 23 (12): e76-e82, 2017.[PUBMED Abstract]
- Schultz KAP, Williams GM, Kamihara J, et al.: DICER1 and Associated Conditions: Identification of At-risk Individuals and Recommended Surveillance Strategies. Clin Cancer Res 24 (10): 2251-2261, 2018.[PUBMED Abstract]
- Mitchell SG, Pencheva B, Porter CC: Germline Genetics and Childhood Cancer: Emerging Cancer Predisposition Syndromes and Psychosocial Impacts. Curr Oncol Rep 21 (10): 85, 2019.[PUBMED Abstract]
- Green DM: Wilms' tumor. In: Greem DM: Diagnosis and Management of Malignant Solid Tumors in Infants and Children. Boston, Ma: Martinus Nijhoff Publishing, 1985, pp 129-86.[PUBMED Abstract]
- Servaes S, Khanna G, Naranjo A, et al.: Comparison of diagnostic performance of CT and MRI for abdominal staging of pediatric renal tumors: a report from the Children's Oncology Group. Pediatr Radiol 45 (2): 166-72, 2015.[PUBMED Abstract]
- Khanna G, Naranjo A, Hoffer F, et al.: Detection of preoperative wilms tumor rupture with CT: a report from the Children's Oncology Group. Radiology 266 (2): 610-7, 2013.[PUBMED Abstract]
- McDonald K, Duffy P, Chowdhury T, et al.: Added value of abdominal cross-sectional imaging (CT or MRI) in staging of Wilms' tumours. Clin Radiol 68 (1): 16-20, 2013.[PUBMED Abstract]
- Ritchey ML, Shamberger RC, Hamilton T, et al.: Fate of bilateral renal lesions missed on preoperative imaging: a report from the National Wilms Tumor Study Group. J Urol 174 (4 Pt 2): 1519-21; discussion 1521, 2005.[PUBMED Abstract]
- Khanna G, Rosen N, Anderson JR, et al.: Evaluation of diagnostic performance of CT for detection of tumor thrombus in children with Wilms tumor: a report from the Children's Oncology Group. Pediatr Blood Cancer 58 (4): 551-5, 2012.[PUBMED Abstract]
- Begent J, Sebire NJ, Levitt G, et al.: Pilot study of F(18)-Fluorodeoxyglucose Positron Emission Tomography/computerised tomography in Wilms' tumour: correlation with conventional imaging, pathology and immunohistochemistry. Eur J Cancer 47 (3): 389-96, 2011.[PUBMED Abstract]
- Callaghan MU, Wong TE, Federici AB: Treatment of acquired von Willebrand syndrome in childhood. Blood 122 (12): 2019-22, 2013.[PUBMED Abstract]
- Shamberger RC, Guthrie KA, Ritchey ML, et al.: Surgery-related factors and local recurrence of Wilms tumor in National Wilms Tumor Study 4. Ann Surg 229 (2): 292-7, 1999.[PUBMED Abstract]
- Ehrlich P, Chi YY, Chintagumpala MM, et al.: Results of the First Prospective Multi-institutional Treatment Study in Children With Bilateral Wilms Tumor (AREN0534): A Report From the Children's Oncology Group. Ann Surg 266 (3): 470-478, 2017.[PUBMED Abstract]
- Hamilton TE, Green DM, Perlman EJ, et al.: Bilateral Wilms' tumor with anaplasia: lessons from the National Wilms' Tumor Study. J Pediatr Surg 41 (10): 1641-4, 2006.[PUBMED Abstract]
- Othersen HB, DeLorimer A, Hrabovsky E, et al.: Surgical evaluation of lymph node metastases in Wilms' tumor. J Pediatr Surg 25 (3): 330-1, 1990.[PUBMED Abstract]
- Green DM: Controversies in the management of Wilms tumour - immediate nephrectomy or delayed nephrectomy? Eur J Cancer 43 (17): 2453-6, 2007.[PUBMED Abstract]
- Shamberger RC, Ritchey ML, Haase GM, et al.: Intravascular extension of Wilms tumor. Ann Surg 234 (1): 116-21, 2001.[PUBMED Abstract]
- Servaes SE, Hoffer FA, Smith EA, et al.: Imaging of Wilms tumor: an update. Pediatr Radiol 49 (11): 1441-1452, 2019.[PUBMED Abstract]
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014.[PUBMED Abstract]
- Green DM, Breslow NE, Beckwith JB, et al.: Effect of duration of treatment on treatment outcome and cost of treatment for Wilms' tumor: a report from the National Wilms' Tumor Study Group. J Clin Oncol 16 (12): 3744-51, 1998.[PUBMED Abstract]
- Kalapurakal JA, Dome JS, Perlman EJ, et al.: Management of Wilms' tumour: current practice and future goals. Lancet Oncol 5 (1): 37-46, 2004.[PUBMED Abstract]
- Ehrlich PF: Wilms tumor: progress and considerations for the surgeon. Surg Oncol 16 (3): 157-71, 2007.[PUBMED Abstract]
- Dome JS, Cotton CA, Perlman EJ, et al.: Treatment of anaplastic histology Wilms' tumor: results from the fifth National Wilms' Tumor Study. J Clin Oncol 24 (15): 2352-8, 2006.[PUBMED Abstract]
- Shamberger RC, Anderson JR, Breslow NE, et al.: Long-term outcomes for infants with very low risk Wilms tumor treated with surgery alone in National Wilms Tumor Study-5. Ann Surg 251 (3): 555-8, 2010.[PUBMED Abstract]
- Hol JA, Lopez-Yurda MI, Van Tinteren H, et al.: Prognostic significance of age in 5631 patients with Wilms tumour prospectively registered in International Society of Paediatric Oncology (SIOP) 93-01 and 2001. PLoS One 14 (8): e0221373, 2019.[PUBMED Abstract]
- Mitry E, Ciccolallo L, Coleman MP, et al.: Incidence of and survival from Wilms' tumour in adults in Europe: data from the EUROCARE study. Eur J Cancer 42 (14): 2363-8, 2006.[PUBMED Abstract]
- Chen I, Pasalic D, Fischer-Valuck B, et al.: Disparity in Outcomes for Adolescent and Young Adult Patients Diagnosed With Pediatric Solid Tumors Across 4 Decades. Am J Clin Oncol 41 (5): 471-475, 2018.[PUBMED Abstract]
- Kalapurakal JA, Nan B, Norkool P, et al.: Treatment outcomes in adults with favorable histologic type Wilms tumor-an update from the National Wilms Tumor Study Group. Int J Radiat Oncol Biol Phys 60 (5): 1379-84, 2004.[PUBMED Abstract]
- Arrigo S, Beckwith JB, Sharples K, et al.: Better survival after combined modality care for adults with Wilms' tumor. A report from the National Wilms' Tumor Study. Cancer 66 (5): 827-30, 1990.[PUBMED Abstract]
- Byrd RL, Evans AE, D'Angio GJ: Adult Wilms tumor: effect of combined therapy on survival. J Urol 127 (4): 648-51, 1982.[PUBMED Abstract]
- de Vries-Brilland M, Sionneau B, Dutriaux C, et al.: Successful Treatment of Metastatic Adult Wilms Tumor With Anti-BRAF Treatment: A Case Report and a Brief Review of the Literature. Clin Genitourin Cancer 17 (4): e721-e723, 2019.[PUBMED Abstract]
- Segers H, van den Heuvel-Eibrink MM, Pritchard-Jones K, et al.: Management of adults with Wilms' tumor: recommendations based on international consensus. Expert Rev Anticancer Ther 11 (7): 1105-13, 2011.[PUBMED Abstract]
- Perlman EJ: Pediatric renal tumors: practical updates for the pathologist. Pediatr Dev Pathol 8 (3): 320-38, 2005 May-Jun.[PUBMED Abstract]
- Popov SD, Sebire NJ, Pritchard-Jones K, et al.: Renal tumors in children aged 10-16 Years: a report from the United Kingdom Children's Cancer and Leukaemia Group. Pediatr Dev Pathol 14 (3): 189-93, 2011 May-Jun.[PUBMED Abstract]
- Indolfi P, Jenkner A, Terenziani M, et al.: Synchronous bilateral Wilms tumor: a report from the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP). Cancer 119 (8): 1586-92, 2013.[PUBMED Abstract]
- Hamilton TE, Ritchey ML, Haase GM, et al.: The management of synchronous bilateral Wilms tumor: a report from the National Wilms Tumor Study Group. Ann Surg 253 (5): 1004-10, 2011.[PUBMED Abstract]
- Williams RD, Al-Saadi R, Natrajan R, et al.: Molecular profiling reveals frequent gain of MYCN and anaplasia-specific loss of 4q and 14q in Wilms tumor. Genes Chromosomes Cancer 50 (12): 982-95, 2011.[PUBMED Abstract]
- Vujanić GM, Harms D, Sandstedt B, et al.: New definitions of focal and diffuse anaplasia in Wilms tumor: the International Society of Paediatric Oncology (SIOP) experience. Med Pediatr Oncol 32 (5): 317-23, 1999.[PUBMED Abstract]
- Faria P, Beckwith JB, Mishra K, et al.: Focal versus diffuse anaplasia in Wilms tumor--new definitions with prognostic significance: a report from the National Wilms Tumor Study Group. Am J Surg Pathol 20 (8): 909-20, 1996.[PUBMED Abstract]
- Beckwith JB: New developments in the pathology of Wilms tumor. Cancer Invest 15 (2): 153-62, 1997.[PUBMED Abstract]
- Beckwith JB: Precursor lesions of Wilms tumor: clinical and biological implications. Med Pediatr Oncol 21 (3): 158-68, 1993.[PUBMED Abstract]
- Perlman EJ, Faria P, Soares A, et al.: Hyperplastic perilobar nephroblastomatosis: long-term survival of 52 patients. Pediatr Blood Cancer 46 (2): 203-21, 2006.[PUBMED Abstract]
- Furtwängler R, Schmolze M, Gräber S, et al.: Pretreatment for bilateral nephroblastomatosis is an independent risk factor for progressive disease in patients with stage V nephroblastoma. Klin Padiatr 226 (3): 175-81, 2014.[PUBMED Abstract]
- Cooke A, Deshpande AV, La Hei ER, et al.: Ectopic nephrogenic rests in children: the clinicosurgical implications. J Pediatr Surg 44 (12): e13-6, 2009.[PUBMED Abstract]
- Wilms' tumor: status report, 1990. By the National Wilms' Tumor Study Committee. J Clin Oncol 9 (5): 877-87, 1991.[PUBMED Abstract]
- Green DM, Breslow NE, D'Angio GJ, et al.: Outcome of patients with Stage II/favorable histology Wilms tumor with and without local tumor spill: a report from the National Wilms Tumor Study Group. Pediatr Blood Cancer 61 (1): 134-9, 2014.[PUBMED Abstract]
- Ehrlich PF, Anderson JR, Ritchey ML, et al.: Clinicopathologic findings predictive of relapse in children with stage III favorable-histology Wilms tumor. J Clin Oncol 31 (9): 1196-201, 2013.[PUBMED Abstract]
- D'Angio GJ, Breslow N, Beckwith JB, et al.: Treatment of Wilms' tumor. Results of the Third National Wilms' Tumor Study. Cancer 64 (2): 349-60, 1989.[PUBMED Abstract]
- Jereb B, Burgers JM, Tournade MF, et al.: Radiotherapy in the SIOP (International Society of Pediatric Oncology) nephroblastoma studies: a review. Med Pediatr Oncol 22 (4): 221-7, 1994.[PUBMED Abstract]
- Green DM: The treatment of stages I-IV favorable histology Wilms' tumor. J Clin Oncol 22 (8): 1366-72, 2004.[PUBMED Abstract]
- Graf N, Tournade MF, de Kraker J: The role of preoperative chemotherapy in the management of Wilms' tumor. The SIOP studies. International Society of Pediatric Oncology. Urol Clin North Am 27 (3): 443-54, 2000.[PUBMED Abstract]
- Vujanić GM, D'Hooghe E, Popov SD, et al.: The effect of preoperative chemotherapy on histological subtyping and staging of Wilms tumors: The United Kingdom Children's Cancer Study Group (UKCCSG) Wilms tumor trial 3 (UKW3) experience. Pediatr Blood Cancer 66 (3): e27549, 2019.[PUBMED Abstract]
- van den Heuvel-Eibrink MM, Hol JA, Pritchard-Jones K, et al.: Position paper: Rationale for the treatment of Wilms tumour in the UMBRELLA SIOP-RTSG 2016 protocol. Nat Rev Urol 14 (12): 743-752, 2017.[PUBMED Abstract]
- Green DM, Breslow NE, Beckwith JB, et al.: Comparison between single-dose and divided-dose administration of dactinomycin and doxorubicin for patients with Wilms' tumor: a report from the National Wilms' Tumor Study Group. J Clin Oncol 16 (1): 237-45, 1998.[PUBMED Abstract]
- D'Angio GJ, Evans AE, Breslow N, et al.: The treatment of Wilms' tumor: Results of the national Wilms' tumor study. Cancer 38 (2): 633-46, 1976.[PUBMED Abstract]
- D'Angio GJ, Evans A, Breslow N, et al.: The treatment of Wilms' tumor: results of the Second National Wilms' Tumor Study. Cancer 47 (9): 2302-11, 1981.[PUBMED Abstract]
- Kieran K, Anderson JR, Dome JS, et al.: Lymph node involvement in Wilms tumor: results from National Wilms Tumor Studies 4 and 5. J Pediatr Surg 47 (4): 700-6, 2012.[PUBMED Abstract]
- Ritchey M, Daley S, Shamberger RC, et al.: Ureteral extension in Wilms' tumor: a report from the National Wilms' Tumor Study Group (NWTSG). J Pediatr Surg 43 (9): 1625-9, 2008.[PUBMED Abstract]
- Gow KW, Barnhart DC, Hamilton TE, et al.: Primary nephrectomy and intraoperative tumor spill: report from the Children's Oncology Group (COG) renal tumors committee. J Pediatr Surg 48 (1): 34-8, 2013.[PUBMED Abstract]
- McNeil DE, Langer JC, Choyke P, et al.: Feasibility of partial nephrectomy for Wilms' tumor in children with Beckwith-Wiedemann syndrome who have been screened with abdominal ultrasonography. J Pediatr Surg 37 (1): 57-60, 2002.[PUBMED Abstract]
- Scalabre A, Bergeron C, Brioude F, et al.: Is Nephron Sparing Surgery Justified in Wilms Tumor With Beckwith-Wiedemann Syndrome or Isolated Hemihypertrophy? Pediatr Blood Cancer 63 (9): 1571-7, 2016.[PUBMED Abstract]
- Auber F, Jeanpierre C, Denamur E, et al.: Management of Wilms tumors in Drash and Frasier syndromes. Pediatr Blood Cancer 52 (1): 55-9, 2009.[PUBMED Abstract]
- Neville H, Ritchey ML, Shamberger RC, et al.: The occurrence of Wilms tumor in horseshoe kidneys: a report from the National Wilms Tumor Study Group (NWTSG). J Pediatr Surg 37 (8): 1134-7, 2002.[PUBMED Abstract]
- Ferrer FA, Rosen N, Herbst K, et al.: Image based feasibility of renal sparing surgery for very low risk unilateral Wilms tumors: a report from the Children's Oncology Group. J Urol 190 (5): 1846-51, 2013.[PUBMED Abstract]
- Ritchey ML: Renal sparing surgery for Wilms tumor. J Urol 174 (4 Pt 1): 1172-3, 2005.[PUBMED Abstract]
- Cozzi DA, Zani A: Nephron-sparing surgery in children with primary renal tumor: indications and results. Semin Pediatr Surg 15 (1): 3-9, 2006.[PUBMED Abstract]
- Zhuge Y, Cheung MC, Yang R, et al.: Improved survival with lymph node sampling in Wilms tumor. J Surg Res 167 (2): e199-203, 2011.[PUBMED Abstract]
- Ritchey ML, Kelalis PP, Breslow N, et al.: Surgical complications after nephrectomy for Wilms' tumor. Surg Gynecol Obstet 175 (6): 507-14, 1992.[PUBMED Abstract]
- Ehrlich PF, Ferrer FA, Ritchey ML, et al.: Hepatic metastasis at diagnosis in patients with Wilms tumor is not an independent adverse prognostic factor for stage IV Wilms tumor: a report from the Children's Oncology Group/National Wilms Tumor Study Group. Ann Surg 250 (4): 642-8, 2009.[PUBMED Abstract]
- Ritchey ML: Primary nephrectomy for Wilms' tumor: approach of the National Wilms' Tumor Study Group. Urology 47 (6): 787-91, 1996.[PUBMED Abstract]
- Lall A, Pritchard-Jones K, Walker J, et al.: Wilms' tumor with intracaval thrombus in the UK Children's Cancer Study Group UKW3 trial. J Pediatr Surg 41 (2): 382-7, 2006.[PUBMED Abstract]
- Ritchey ML, Pringle KC, Breslow NE, et al.: Management and outcome of inoperable Wilms tumor. A report of National Wilms Tumor Study-3. Ann Surg 220 (5): 683-90, 1994.[PUBMED Abstract]
- Ritchey ML, Shamberger RC, Haase G, et al.: Surgical complications after primary nephrectomy for Wilms' tumor: report from the National Wilms' Tumor Study Group. J Am Coll Surg 192 (1): 63-8; quiz 146, 2001.[PUBMED Abstract]
- Tournade MF, Com-Nougué C, Voûte PA, et al.: Results of the Sixth International Society of Pediatric Oncology Wilms' Tumor Trial and Study: a risk-adapted therapeutic approach in Wilms' tumor. J Clin Oncol 11 (6): 1014-23, 1993.[PUBMED Abstract]
- Oberholzer HF, Falkson G, De Jager LC: Successful management of inferior vena cava and right atrial nephroblastoma tumor thrombus with preoperative chemotherapy. Med Pediatr Oncol 20 (1): 61-3, 1992.[PUBMED Abstract]
- Saarinen UM, Wikström S, Koskimies O, et al.: Percutaneous needle biopsy preceding preoperative chemotherapy in the management of massive renal tumors in children. J Clin Oncol 9 (3): 406-15, 1991.[PUBMED Abstract]
- Dykes EH, Marwaha RK, Dicks-Mireaux C, et al.: Risks and benefits of percutaneous biopsy and primary chemotherapy in advanced Wilms' tumour. J Pediatr Surg 26 (5): 610-2, 1991.[PUBMED Abstract]
- Thompson WR, Newman K, Seibel N, et al.: A strategy for resection of Wilms' tumor with vena cava or atrial extension. J Pediatr Surg 27 (7): 912-5, 1992.[PUBMED Abstract]
- Szavay P, Luithle T, Semler O, et al.: Surgery of cavoatrial tumor thrombus in nephroblastoma: a report of the SIOP/GPOH study. Pediatr Blood Cancer 43 (1): 40-5, 2004.[PUBMED Abstract]
- Powis M, Messahel B, Hobson R, et al.: Surgical complications after immediate nephrectomy versus preoperative chemotherapy in non-metastatic Wilms' tumour: findings from the 1991-2001 United Kingdom Children's Cancer Study Group UKW3 Trial. J Pediatr Surg 48 (11): 2181-6, 2013.[PUBMED Abstract]
- Rutigliano DN, Kayton ML, Steinherz P, et al.: The use of preoperative chemotherapy in Wilms' tumor with contained retroperitoneal rupture. J Pediatr Surg 42 (9): 1595-9, 2007.[PUBMED Abstract]
- Brisse HJ, Schleiermacher G, Sarnacki S, et al.: Preoperative Wilms tumor rupture: a retrospective study of 57 patients. Cancer 113 (1): 202-13, 2008.[PUBMED Abstract]
- Corn BW, Goldwein JW, Evans I, et al.: Outcomes in low-risk babies treated with half-dose chemotherapy according to the Third National Wilms' Tumor Study. J Clin Oncol 10 (8): 1305-9, 1992.[PUBMED Abstract]
- Morgan E, Baum E, Breslow N, et al.: Chemotherapy-related toxicity in infants treated according to the Second National Wilms' Tumor Study. J Clin Oncol 6 (1): 51-5, 1988.[PUBMED Abstract]
- Green DM, Norkool P, Breslow NE, et al.: Severe hepatic toxicity after treatment with vincristine and dactinomycin using single-dose or divided-dose schedules: a report from the National Wilms' Tumor Study. J Clin Oncol 8 (9): 1525-30, 1990.[PUBMED Abstract]
- Raine J, Bowman A, Wallendszus K, et al.: Hepatopathy-thrombocytopenia syndrome--a complication of dactinomycin therapy for Wilms' tumor: a report from the United Kingdom Childrens Cancer Study Group. J Clin Oncol 9 (2): 268-73, 1991.[PUBMED Abstract]
- Feusner JH, Ritchey ML, Norkool PA, et al.: Renal failure does not preclude cure in children receiving chemotherapy for Wilms tumor: a report from the National Wilms Tumor Study Group. Pediatr Blood Cancer 50 (2): 242-5, 2008.[PUBMED Abstract]
- Veal GJ, English MW, Grundy RG, et al.: Pharmacokinetically guided dosing of carboplatin in paediatric cancer patients with bilateral nephrectomy. Cancer Chemother Pharmacol 54 (4): 295-300, 2004.[PUBMED Abstract]
- Dix DB, Fernandez CV, Chi YY, et al.: Augmentation of Therapy for Combined Loss of Heterozygosity 1p and 16q in Favorable Histology Wilms Tumor: A Children's Oncology Group AREN0532 and AREN0533 Study Report. J Clin Oncol 37 (30): 2769-2777, 2019.[PUBMED Abstract]
- Stokes CL, Stokes WA, Kalapurakal JA, et al.: Timing of Radiation Therapy in Pediatric Wilms Tumor: A Report From the National Cancer Database. Int J Radiat Oncol Biol Phys 101 (2): 453-461, 2018.[PUBMED Abstract]
- Dix DB, Seibel NL, Chi YY, et al.: Treatment of Stage IV Favorable Histology Wilms Tumor With Lung Metastases: A Report From the Children's Oncology Group AREN0533 Study. J Clin Oncol 36 (16): 1564-1570, 2018.[PUBMED Abstract]
- Daw NC, Chi YY, Kalapurakal JA, et al.: Activity of Vincristine and Irinotecan in Diffuse Anaplastic Wilms Tumor and Therapy Outcomes of Stage II to IV Disease: Results of the Children's Oncology Group AREN0321 Study. J Clin Oncol 38 (14): 1558-1568, 2020.[PUBMED Abstract]
- Fernandez CV, Perlman EJ, Mullen EA, et al.: Clinical Outcome and Biological Predictors of Relapse After Nephrectomy Only for Very Low-risk Wilms Tumor: A Report From Children's Oncology Group AREN0532. Ann Surg 265 (4): 835-840, 2017.[PUBMED Abstract]
- Thomas PR, Tefft M, Compaan PJ, et al.: Results of two radiation therapy randomizations in the third National Wilms' Tumor Study. Cancer 68 (8): 1703-7, 1991.[PUBMED Abstract]
- Tefft M, D'Angio GJ, Beckwith B, et al.: Patterns of intra-abdominal relapse (IAR) in patients with Wilms' tumor who received radiation: analysis by histopathology. A report of National Wilms' Tumor Studies 1 and 2 (NWTS-1 & 2). Int J Radiat Oncol Biol Phys 6 (6): 663-7, 1980.[PUBMED Abstract]
- Thomas PR, Tefft M, Farewell VT, et al.: Abdominal relapses in irradiated second National Wilms' Tumor Study patients. J Clin Oncol 2 (10): 1098-101, 1984.[PUBMED Abstract]
- Meisel JA, Guthrie KA, Breslow NE, et al.: Significance and management of computed tomography detected pulmonary nodules: a report from the National Wilms Tumor Study Group. Int J Radiat Oncol Biol Phys 44 (3): 579-85, 1999.[PUBMED Abstract]
- Daw NC, Chi YY, Kim Y, et al.: Treatment of stage I anaplastic Wilms' tumour: a report from the Children's Oncology Group AREN0321 study. Eur J Cancer 118: 58-66, 2019.[PUBMED Abstract]
- Green DM, Breslow NE, Beckwith JB, et al.: Treatment with nephrectomy only for small, stage I/favorable histology Wilms' tumor: a report from the National Wilms' Tumor Study Group. J Clin Oncol 19 (17): 3719-24, 2001.[PUBMED Abstract]
- Parsons LN, Mullen EA, Geller JI, et al.: Outcome analysis of stage I epithelial-predominant favorable-histology Wilms tumors: A report from Children's Oncology Group study AREN03B2. Cancer 126 (12): 2866-2871, 2020.[PUBMED Abstract]
- Kalapurakal JA, Li SM, Breslow NE, et al.: Intraoperative spillage of favorable histology wilms tumor cells: influence of irradiation and chemotherapy regimens on abdominal recurrence. A report from the National Wilms Tumor Study Group. Int J Radiat Oncol Biol Phys 76 (1): 201-6, 2010.[PUBMED Abstract]
- Fernandez CV, Mullen EA, Chi YY, et al.: Outcome and Prognostic Factors in Stage III Favorable-Histology Wilms Tumor: A Report From the Children's Oncology Group Study AREN0532. J Clin Oncol 36 (3): 254-261, 2018.[PUBMED Abstract]
- Grundy PE, Green DM, Dirks AC, et al.: Clinical significance of pulmonary nodules detected by CT and Not CXR in patients treated for favorable histology Wilms tumor on national Wilms tumor studies-4 and -5: a report from the Children's Oncology Group. Pediatr Blood Cancer 59 (4): 631-5, 2012.[PUBMED Abstract]
- Verschuur A, Van Tinteren H, Graf N, et al.: Treatment of pulmonary metastases in children with stage IV nephroblastoma with risk-based use of pulmonary radiotherapy. J Clin Oncol 30 (28): 3533-9, 2012.[PUBMED Abstract]
- Breslow NE, Collins AJ, Ritchey ML, et al.: End stage renal disease in patients with Wilms tumor: results from the National Wilms Tumor Study Group and the United States Renal Data System. J Urol 174 (5): 1972-5, 2005.[PUBMED Abstract]
- Aronson DC, Slaar A, Heinen RC, et al.: Long-term outcome of bilateral Wilms tumors (BWT). Pediatr Blood Cancer 56 (7): 1110-3, 2011.[PUBMED Abstract]
- Zuppan CW, Beckwith JB, Weeks DA, et al.: The effect of preoperative therapy on the histologic features of Wilms' tumor. An analysis of cases from the Third National Wilms' Tumor Study. Cancer 68 (2): 385-94, 1991.[PUBMED Abstract]
- Ehrlich PF: Bilateral Wilms' tumor: the need to improve outcomes. Expert Rev Anticancer Ther 9 (7): 963-73, 2009.[PUBMED Abstract]
- Sudour H, Audry G, Schleimacher G, et al.: Bilateral Wilms tumors (WT) treated with the SIOP 93 protocol in France: epidemiological survey and patient outcome. Pediatr Blood Cancer 59 (1): 57-61, 2012.[PUBMED Abstract]
- Davidoff AM, Interiano RB, Wynn L, et al.: Overall Survival and Renal Function of Patients With Synchronous Bilateral Wilms Tumor Undergoing Surgery at a Single Institution. Ann Surg 262 (4): 570-6, 2015.[PUBMED Abstract]
- Kieran K, Williams MA, McGregor LM, et al.: Repeat nephron-sparing surgery for children with bilateral Wilms tumor. J Pediatr Surg 49 (1): 149-53, 2014.[PUBMED Abstract]
- Kist-van Holthe JE, Ho PL, Stablein D, et al.: Outcome of renal transplantation for Wilms' tumor and Denys-Drash syndrome: a report of the North American Pediatric Renal Transplant Cooperative Study. Pediatr Transplant 9 (3): 305-10, 2005.[PUBMED Abstract]
- Wong KF, Reulen RC, Winter DL, et al.: Risk of Adverse Health and Social Outcomes Up to 50 Years After Wilms Tumor: The British Childhood Cancer Survivor Study. J Clin Oncol 34 (15): 1772-9, 2016.[PUBMED Abstract]
- Breslow NE, Lange JM, Friedman DL, et al.: Secondary malignant neoplasms after Wilms tumor: an international collaborative study. Int J Cancer 127 (3): 657-66, 2010.[PUBMED Abstract]
- Termuhlen AM, Tersak JM, Liu Q, et al.: Twenty-five year follow-up of childhood Wilms tumor: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer 57 (7): 1210-6, 2011.[PUBMED Abstract]
- Lange JM, Takashima JR, Peterson SM, et al.: Breast cancer in female survivors of Wilms tumor: a report from the national Wilms tumor late effects study. Cancer 120 (23): 3722-30, 2014.[PUBMED Abstract]
- Green DM, Grigoriev YA, Nan B, et al.: Congestive heart failure after treatment for Wilms' tumor: a report from the National Wilms' Tumor Study group. J Clin Oncol 19 (7): 1926-34, 2001.[PUBMED Abstract]
- Green DM, Lange JM, Peabody EM, et al.: Pregnancy outcome after treatment for Wilms tumor: a report from the national Wilms tumor long-term follow-up study. J Clin Oncol 28 (17): 2824-30, 2010.[PUBMED Abstract]
-
Beckwith-Wiedemann syndrome. Beckwith-Wiedemann syndrome is an overgrowth syndrome characterized by asymmetric growth of one or more parts of the body, large tongue, omphalocele or umbilical hernia at birth, creases or pits in the skin near the ears, kidney abnormalities, and hypoglycemia (in neonates). It is also characterized by the development of Wilms tumor, rhabdomyosarcoma, and hepatoblastoma in the first decade of life. Approximately 15% of children with Beckwith-Wiedemann syndrome will have bilateral tumors.[
20
]
- Renal Cell Carcinoma (RCC)
-
Incidence of RCC
Malignant epithelial tumors arising in the kidneys of children account for more than 5% of new pediatric renal tumors; therefore, they are more common than clear cell sarcoma of the kidney or rhabdoid tumors of the kidney. The annual incidence rate is approximately 4 cases per 1 million children, compared with an incidence of Wilms tumor of the kidney that is at least 29-fold higher.[ 1 ]
RCC, the most common primary malignancy of the kidney in adults, is rare in children younger than 15 years. In the older age group of adolescents (aged 15–19 years), approximately two-thirds of renal malignancies are RCC.[ 2 ] Children and adolescents with RCC (n = 515) present with more advanced disease than do those aged 21 to 30 years.[ 1 ]
Conditions Associated With RCC
Conditions associated with RCC include the following:
-
von Hippel-Lindau (VHL) disease. VHL disease is an autosomal dominant condition in which blood vessels in the retina and cerebellum grow excessively.[
3
] The gene for VHL disease is located on chromosome 3p26 and is a tumor-suppressor gene, which is either mutated or deleted in patients with the syndrome.
Screening for the VHL gene is available.[ 4 ] To detect clear cell renal carcinoma in these individuals when the lesions are smaller than 3 cm and renal-sparing surgery can be performed, annual screening with abdominal ultrasonography or magnetic resonance imaging (MRI) is recommended, beginning at age 8 to 11 years.[ 5 ]
(Refer to the Von Hippel-Lindau Disease section in the PDQ summary on Genetics of Kidney Cancer [Renal Cell Cancer] for more information.)
- Tuberous sclerosis. In tuberous sclerosis, the renal lesions may actually be epithelioid angiomyolipoma (also called perivascular epithelioid cell tumor or PEComa), which is associated with aggressive or malignant behavior and expresses melanocyte and smooth muscle markers.[ 6 ][ 7 ]
-
Familial RCC. Familial RCC has been associated with an inherited chromosome translocation involving chromosome 3.[
8
] A high incidence of chromosome 3 abnormalities has also been demonstrated in nonfamilial renal tumors.
Succinate dehydrogenase (SDHB, SDHC, and SDHD) is a Krebs cycle enzyme gene that has been associated with the development of familial RCC occurring with pheochromocytoma/paraganglioma. Germline mutations in a subunit of the gene have been reported in individuals with renal cancer and no history of pheochromocytoma.[ 9 ][ 10 ]
- Renal medullary carcinoma. A rare subtype of RCC, renal medullary carcinoma may be associated with sickle cell hemoglobinopathy.[ 11 ] Renal medullary carcinomas are highly aggressive malignancies characterized clinically by a high stage at the time of detection, with widespread metastases and lack of response to chemotherapy and radiation therapy.[ 12 ][ 13 ][ Level of evidence: 3iiA] Survival is poor and ranges from 2 weeks to 15 months, with a mean survival of 4 months.[ 11 ][ 13 ][ 14 ][ 15 ][ 16 ]
- Hereditary leiomyomatosis. Hereditary leiomyomatosis (of skin and uterus) and RCC is a distinct phenotype caused by dominant inheritance of a mutation in the FH gene. Screening for RCC starting as early as age 5 years has been recommended.[ 17 ][ 18 ]
- Previous treatment for childhood cancer. Survivors of childhood cancer who were treated with radiation and/or chemotherapy are at more risk of developing renal cancers than are the general population. Highest risk has been observed among neuroblastoma survivors, with renal-directed radiation therapy of 5 Gy or more, and with platinum-based chemotherapy.[ 19 ] Renal cancers have also been reported after treatment for rhabdomyosarcoma, leiomyosarcoma, acute lymphoblastic leukemia, primitive neuroectodermal tumors (PNET), and Wilms tumor.[ 20 ][ 21 ][ 22 ][ 23 ][ 24 ][ 25 ] (Refer to the Subsequent Neoplasms section in the PDQ summary on Late Effects of Treatment for Childhood Cancer for more information.)
Genetic Testing for Children and Adolescents With RCC
Indications for germline genetic testing of children and adolescents with RCC to check for a related syndrome are described in Table 8.
Table 8. Indications for Germline Genetic Analysis (Screening) of Children and Adolescents with Renal Cell Carcinoma (RCC)a Indication for Testing Tumor Histology Gene Test Related Syndrome VHL = von Hippel-Lindau. aAdapted from Linehan et al.[ 26 ] Multifocal RCC or VHL lesions Clear cell VHL gene von Hippel-Lindau syndrome Family history of clear cell RCC or multifocal RCC with absent VHL mutation Clear cell Chromosome 3 gene translocations Hereditary non-VHL clear cell RCC syndrome Multifocal papillary RCC or family history of papillary RCC Papillary MET gene Hereditary papillary RCC syndrome Multifocal RCC or cutaneous fibrofolliculoma or pulmonary cysts or spontaneous pneumothorax Chromophobe or oncocytic or clear cell Germline sequence BHD gene Birt-Hogg-Dubé syndrome Personal or family history of early-onset uterine leiomyomata or cutaneous leiomyomata Type 2 papillary or collecting duct carcinoma FH gene Hereditary leiomyomata/RCC syndrome Multifocal RCC or early-onset RCC or presence of paraganglioma/pheochromocytoma or family history of paraganglioma/pheochromocytoma Clear cell or chromophobe SDHB gene, SDHC gene, SDHD gene Hereditary paraganglioma/pheochromocytoma syndrome Genomics of RCC
Translocation-positive carcinomas of the kidney are recognized as a distinct form of renal cell carcinoma (RCC) and may be the most common form of RCC in children, accounting for 40% to 50% of pediatric RCC.[ 27 ] In a Children's Oncology Group (COG) prospective clinical trial of 120 childhood and adolescent patients with RCC, nearly one-half of patients had translocation-positive RCC.[ 28 ][ 29 ] These carcinomas are characterized by translocations involving the TFE3 gene located on Xp11.2. The TFE3 gene may partner with one of the following genes:
- ASPSCR in t(X;17)(p11.2;q25).
- PRCC in t(X;1)(p11.2;q21).
- SFPQ in t(X;1)(p11.2;p34).
- NONO in inv(X;p11.2;q12).
- CLTC in t(X;17)(p11;q23).
Another less-common translocation subtype, t(6;11)(p21;q12), involving a TFEB gene fusion, induces overexpression of TFEB. The translocations involving TFE3 and TFEB induce overexpression of these proteins, which can be identified by immunohistochemistry.[ 30 ]
Previous exposure to chemotherapy is the only known risk factor for the development of Xp11 translocation RCCs. In one study, the postchemotherapy interval ranged from 4 to 13 years. All reported patients received either a DNA topoisomerase II inhibitor and/or an alkylating agent.[ 31 ][ 32 ]
Controversy exists as to the biological behavior of translocation RCC in children and young adults. Whereas some series have suggested a good prognosis when RCC is treated with surgery alone despite presenting at a more advanced stage (III/IV) than translocation-associated RCC, a meta-analysis reported that these patients have poorer outcomes.[ 33 ][ 34 ][ 35 ] The outcomes for these patients are being studied in the ongoing COG AREN03B2 (NCT00898365) biology and classification study. Vascular endothelial growth factor receptor–targeted therapies and mammalian target of rapamycin (mTOR) inhibitors seem to be active in Xp11 translocation metastatic RCC.[ 36 ] Recurrences have been reported 20 to 30 years after initial resection of the translocation-associated RCC.[ 22 ]
Diagnosis of Xp11 translocation RCC needs to be confirmed by a molecular genetic approach, rather than using TFE3 immunohistochemistry alone, because reported cases have lacked the translocation. There is a rare subset of RCC cases that is positive for TFE3 and lack a TFE3 translocation, showing an ALK translocation instead. This subset of cases represents a newly recognized subgroup within RCC that is estimated to involve 15% to 20% of unclassified pediatric RCC. In the eight reported cases in children aged 6 to 16 years, the following was observed:[ 37 ][ 38 ][ 39 ][ 40 ]
- ALK was fused to VCL in a t(2;10)(p23;q22) translocation (n = 3). The VCL translocation cases all occurred in children with sickle cell trait, whereas none of the TMP3 translocation cases did.
- ALK was fused to TPM3 (n = 3).
- ALK was fused to HOOK-1 on 1p32 (n = 1).
- t(1;2) translocation fusing ALK and TMP3 (n = 1).
Histology of RCC
Pediatric RCC differs histologically from the adult counterparts. Although the two main morphological subgroups of papillary and clear cell can be identified, about 25% of RCCs show heterogeneous features that do not fit into either of these categories.[ 3 ] Childhood RCCs are more frequently of the papillary subtype (20%–50% of pediatric RCCs) and can sometimes occur in the setting of Wilms tumor, metanephric adenoma, and metanephric adenofibroma.[ 41 ]
RCC in children and young adults has a different genetic and morphologic spectrum than that seen in older adults.[ 3 ][ 32 ][ 41 ][ 42 ]
Prognosis and Prognostic Factors for RCC
Prognostic factors for RCC include the following:
- Stage of disease.
- Lymph node involvement.
The primary prognostic factor for RCC is stage of disease. In 304 children and adolescents with RCC identified in the National Cancer Data Base, the median age was 13 years; 39% of patients presented with localized stage I disease, 16% with stage II disease, 33% with stage III disease, and 12% with stage IV disease. The 5-year overall survival (OS) rates were 100% for patients with stage I and stage II disease, 71% for stage III disease, and 8% for stage IV disease.[ 43 ] Age and sex had no significant impact on survival. Survival was negatively impacted by increasing tumor size (P < .001), positive nodal status (P = .001), and higher pathologic stage (P < .001).[ 43 ] The data attained in this article from the National Cancer Data Base are limited, as some patient details are not available and follow up is incomplete. Tumor size of 4 cm or smaller may or may not impact survival and local lymph node involvement may not be as significant in children.
An important difference between the outcomes in children and adults with RCC is the prognostic significance of local lymph node involvement. Adults presenting with RCC and involved lymph nodes have a 5-year OS rate of approximately 20%, but the literature suggests that 72% of children with RCC and local lymph node involvement at diagnosis (without distant metastases) survive their disease.[ 27 ] In another series of 49 patients from a population-based cancer registry, the findings were similar. In this series, 33% of the patients had papillary subtype, 22% had translocation type, 6% had clear-cell subtype, and 16% were unclassified. The survival rates at 5 years were 96% for patients with localized disease, 75% for patients with positive regional lymph nodes, and 33% for patients with distant metastatic RCC.[ 44 ]
Clinical Features and Diagnostic Evaluation of RCC
RCC may present with the following:
- Abdominal mass.
- Abdominal pain.
- Hematuria.
Refer to the Clinical Features of Wilms Tumor and Diagnostic and Staging Evaluation for Wilms Tumor sections of this summary for more information about the clinical features and diagnostic evaluation of childhood kidney tumors. In a COG prospective clinical trial of 40 patients with small (7 cm) primary tumors whose lymph nodes were adequately sampled, 19 had positive nodes.[ 28 ] Outcome results of this trial are pending. (Refer to the Stage Information for Renal Cell Cancer section in the PDQ summary on adult Renal Cell Cancer Treatment summary for more information about the staging evaluation.)
Treatment of RCC
Survival of patients with RCC is affected by stage of disease at presentation and the completeness of resection at radical nephrectomy. OS rates for all patients with RCC range from 64% to 87%. The 5-year survival rates for pediatric RCC are 90% or higher for stage I, higher than 80% for stage II, 70% for stage III, and lower than 15% for stage IV.[ 27 ] Retrospective analyses and the small number of patients involved place limitations on the level of evidence in the area of treatment.
Standard treatment options for RCC include the following:
Radical nephrectomy with lymph node dissection
The primary treatment for RCC includes total surgical removal of the kidney and associated lymph nodes.[ 27 ]
Renal-sparing surgery with lymph node dissection
Renal-sparing surgery may be considered for carefully selected patients with low-volume localized disease. In two small series, patients who had partial nephrectomies seemed to have outcomes equivalent to those who had radical nephrectomies.[ 32 ][ 45 ]
Other approaches
As with adult RCC, there is no standard treatment for unresectable metastatic disease in children. The response to radiation is poor, and chemotherapy is not effective. Immunotherapy with such agents as interferon-alpha and interleukin-2 may have some effect on cancer control.[ 46 ][ 47 ] Spontaneous regression of pulmonary metastasis rarely occurs with resection of the primary tumor.
Several targeted therapies (e.g., sorafenib, sunitinib, bevacizumab, temsirolimus, pazopanib, axitinib, and everolimus) have been approved for use in adults with RCC; however, these agents have not been tested in pediatric patients with RCC. Case reports of pediatric and adolescent patients with TFE3 translocation–positive RCC suggest responsiveness to multiple tyrosine kinase inhibitors.[ 29 ][ 48 ][ 49 ] Disease regression and improvement in symptoms have been reported with the use of cabozantinib in pediatric patients with translocation-positive RCC expressing MET.[ 50 ] Any RCC that is positive for TFE3 and lacks a translocation should be tested for ALK expression and translocation. Recognition of this subtype may lead to consideration of ALK inhibitor therapy.[ 37 ]
(Refer to the PDQ summary on adult Renal Cell Cancer Treatment for more information about the use of targeted therapies.)
Treatment Options Under Clinical Evaluation for RCC
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- AREN1721 (NCT03595124) (Axitinib and Nivolumab in Treating Participants With Unresectable or Metastatic TFE/Translocation RCC [tRCC]): TFE/tRCC is a distinct, typically translocation-associated RCC with characteristic morphology and immunohistochemical expression of TFE3 or TFEB. Nearly 50% of pediatric RCCs are tRCC, and it accounts for 1% to 5% of RCCs overall, typically in the adolescent and young adult population. This is the first prospective therapeutic study of patients with tRCC. Patients aged 12 months and older who have histologically confirmed unresectable TFE/tRCC or metastatic TFE/tRCC diagnosed using WHO-defined criteria are eligible. Patients may be newly diagnosed or have received previous cancer therapy and must have measurable disease. Patients will be randomly assigned at the time of enrollment to one of three therapeutic arms: axitinib, nivolumab, or a combination of axitinib and nivolumab.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
参考文献- Akhavan A, Richards M, Shnorhavorian M, et al.: Renal cell carcinoma in children, adolescents and young adults: a National Cancer Database study. J Urol 193 (4): 1336-41, 2015.[PUBMED Abstract]
- Bernstein L, Linet M, Smith MA, et al.: Renal tumors. In: Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. Bethesda, Md: National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649, pp 79-90. Also available online. Last accessed June 08, 2020.[PUBMED Abstract]
- Bruder E, Passera O, Harms D, et al.: Morphologic and molecular characterization of renal cell carcinoma in children and young adults. Am J Surg Pathol 28 (9): 1117-32, 2004.[PUBMED Abstract]
- Schimke RN, Collins DL, Stolle CA: Von Hippel-Lindau syndrome. In: Pagon RA, Adam MP, Bird TD, et al., eds.: GeneReviews. Seattle, Wash: University of Washington, 1993-2018, pp. Available online. Last accessed June 08, 2020.[PUBMED Abstract]
- Teplick A, Kowalski M, Biegel JA, et al.: Educational paper: screening in cancer predisposition syndromes: guidelines for the general pediatrician. Eur J Pediatr 170 (3): 285-94, 2011.[PUBMED Abstract]
- Park HK, Zhang S, Wong MK, et al.: Clinical presentation of epithelioid angiomyolipoma. Int J Urol 14 (1): 21-5, 2007.[PUBMED Abstract]
- Pea M, Bonetti F, Martignoni G, et al.: Apparent renal cell carcinomas in tuberous sclerosis are heterogeneous: the identification of malignant epithelioid angiomyolipoma. Am J Surg Pathol 22 (2): 180-7, 1998.[PUBMED Abstract]
- Wang N, Perkins KL: Involvement of band 3p14 in t(3;8) hereditary renal carcinoma. Cancer Genet Cytogenet 11 (4): 479-81, 1984.[PUBMED Abstract]
- Ricketts C, Woodward ER, Killick P, et al.: Germline SDHB mutations and familial renal cell carcinoma. J Natl Cancer Inst 100 (17): 1260-2, 2008.[PUBMED Abstract]
- Linehan WM, Bratslavsky G, Pinto PA, et al.: Molecular diagnosis and therapy of kidney cancer. Annu Rev Med 61: 329-43, 2010.[PUBMED Abstract]
- Swartz MA, Karth J, Schneider DT, et al.: Renal medullary carcinoma: clinical, pathologic, immunohistochemical, and genetic analysis with pathogenetic implications. Urology 60 (6): 1083-9, 2002.[PUBMED Abstract]
- Sandberg JK, Mullen EA, Cajaiba MM, et al.: Imaging of renal medullary carcinoma in children and young adults: a report from the Children's Oncology Group. Pediatr Radiol 47 (12): 1615-1621, 2017.[PUBMED Abstract]
- Hakimi AA, Koi PT, Milhoua PM, et al.: Renal medullary carcinoma: the Bronx experience. Urology 70 (5): 878-82, 2007.[PUBMED Abstract]
- Strouse JJ, Spevak M, Mack AK, et al.: Significant responses to platinum-based chemotherapy in renal medullary carcinoma. Pediatr Blood Cancer 44 (4): 407-11, 2005.[PUBMED Abstract]
- Rathmell WK, Monk JP: High-dose-intensity MVAC for Advanced Renal Medullary Carcinoma: Report of Three Cases and Literature Review. Urology 72 (3): 659-63, 2008.[PUBMED Abstract]
- Ezekian B, Englum B, Gilmore BF, et al.: Renal medullary carcinoma: A national analysis of 159 patients. Pediatr Blood Cancer 64 (11): , 2017.[PUBMED Abstract]
- Alrashdi I, Levine S, Paterson J, et al.: Hereditary leiomyomatosis and renal cell carcinoma: very early diagnosis of renal cancer in a paediatric patient. Fam Cancer 9 (2): 239-43, 2010.[PUBMED Abstract]
- Bayley JP, Launonen V, Tomlinson IP: The FH mutation database: an online database of fumarate hydratase mutations involved in the MCUL (HLRCC) tumor syndrome and congenital fumarase deficiency. BMC Med Genet 9: 20, 2008.[PUBMED Abstract]
- Wilson CL, Ness KK, Neglia JP, et al.: Renal carcinoma after childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst 105 (7): 504-8, 2013.[PUBMED Abstract]
- Dhall D, Al-Ahmadie HA, Dhall G, et al.: Pediatric renal cell carcinoma with oncocytoid features occurring in a child after chemotherapy for cardiac leiomyosarcoma. Urology 70 (1): 178.e13-5, 2007.[PUBMED Abstract]
- Schafernak KT, Yang XJ, Hsueh W, et al.: Pediatric renal cell carcinoma as second malignancy: reports of two cases and a review of the literature. Can J Urol 14 (6): 3739-44, 2007.[PUBMED Abstract]
- Rais-Bahrami S, Drabick JJ, De Marzo AM, et al.: Xp11 translocation renal cell carcinoma: delayed but massive and lethal metastases of a chemotherapy-associated secondary malignancy. Urology 70 (1): 178.e3-6, 2007.[PUBMED Abstract]
- Brassesco MS, Valera ET, Bonilha TA, et al.: Secondary PSF/TFE3-associated renal cell carcinoma in a child treated for genitourinary rhabdomyosarcoma. Cancer Genet 204 (2): 108-10, 2011.[PUBMED Abstract]
- Breslow NE, Lange JM, Friedman DL, et al.: Secondary malignant neoplasms after Wilms tumor: an international collaborative study. Int J Cancer 127 (3): 657-66, 2010.[PUBMED Abstract]
- Falzarano SM, McKenney JK, Montironi R, et al.: Renal Cell Carcinoma Occurring in Patients With Prior Neuroblastoma: A Heterogenous Group of Neoplasms. Am J Surg Pathol 40 (7): 989-97, 2016.[PUBMED Abstract]
- Linehan WM, Pinto PA, Bratslavsky G, et al.: Hereditary kidney cancer: unique opportunity for disease-based therapy. Cancer 115 (10 Suppl): 2252-61, 2009.[PUBMED Abstract]
- Geller JI, Dome JS: Local lymph node involvement does not predict poor outcome in pediatric renal cell carcinoma. Cancer 101 (7): 1575-83, 2004.[PUBMED Abstract]
- Geller JI, Ehrlich PF, Cost NG, et al.: Characterization of adolescent and pediatric renal cell carcinoma: A report from the Children's Oncology Group study AREN03B2. Cancer 121 (14): 2457-64, 2015.[PUBMED Abstract]
- Ambalavanan M, Geller JI: Treatment of advanced pediatric renal cell carcinoma. Pediatr Blood Cancer 66 (8): e27766, 2019.[PUBMED Abstract]
- Argani P, Hicks J, De Marzo AM, et al.: Xp11 translocation renal cell carcinoma (RCC): extended immunohistochemical profile emphasizing novel RCC markers. Am J Surg Pathol 34 (9): 1295-303, 2010.[PUBMED Abstract]
- Argani P, Laé M, Ballard ET, et al.: Translocation carcinomas of the kidney after chemotherapy in childhood. J Clin Oncol 24 (10): 1529-34, 2006.[PUBMED Abstract]
- Ramphal R, Pappo A, Zielenska M, et al.: Pediatric renal cell carcinoma: clinical, pathologic, and molecular abnormalities associated with the members of the mit transcription factor family. Am J Clin Pathol 126 (3): 349-64, 2006.[PUBMED Abstract]
- Geller JI, Argani P, Adeniran A, et al.: Translocation renal cell carcinoma: lack of negative impact due to lymph node spread. Cancer 112 (7): 1607-16, 2008.[PUBMED Abstract]
- Camparo P, Vasiliu V, Molinie V, et al.: Renal translocation carcinomas: clinicopathologic, immunohistochemical, and gene expression profiling analysis of 31 cases with a review of the literature. Am J Surg Pathol 32 (5): 656-70, 2008.[PUBMED Abstract]
- Qiu Rao, Bing Guan, Zhou XJ: Xp11.2 Translocation renal cell carcinomas have a poorer prognosis than non-Xp11.2 translocation carcinomas in children and young adults: a meta-analysis. Int J Surg Pathol 18 (6): 458-64, 2010.[PUBMED Abstract]
- Malouf GG, Camparo P, Oudard S, et al.: Targeted agents in metastatic Xp11 translocation/TFE3 gene fusion renal cell carcinoma (RCC): a report from the Juvenile RCC Network. Ann Oncol 21 (9): 1834-8, 2010.[PUBMED Abstract]
- Thorner PS, Shago M, Marrano P, et al.: TFE3-positive renal cell carcinomas are not always Xp11 translocation carcinomas: Report of a case with a TPM3-ALK translocation. Pathol Res Pract 212 (10): 937-942, 2016.[PUBMED Abstract]
- Cajaiba MM, Jennings LJ, Rohan SM, et al.: ALK-rearranged renal cell carcinomas in children. Genes Chromosomes Cancer 55 (5): 442-51, 2016.[PUBMED Abstract]
- Smith NE, Deyrup AT, Mariño-Enriquez A, et al.: VCL-ALK renal cell carcinoma in children with sickle-cell trait: the eighth sickle-cell nephropathy? Am J Surg Pathol 38 (6): 858-63, 2014.[PUBMED Abstract]
- Cajaiba MM, Jennings LJ, George D, et al.: Expanding the spectrum of ALK-rearranged renal cell carcinomas in children: Identification of a novel HOOK1-ALK fusion transcript. Genes Chromosomes Cancer 55 (10): 814-7, 2016.[PUBMED Abstract]
- Estrada CR, Suthar AM, Eaton SH, et al.: Renal cell carcinoma: Children's Hospital Boston experience. Urology 66 (6): 1296-300, 2005.[PUBMED Abstract]
- Carcao MD, Taylor GP, Greenberg ML, et al.: Renal-cell carcinoma in children: a different disorder from its adult counterpart? Med Pediatr Oncol 31 (3): 153-8, 1998.[PUBMED Abstract]
- Rialon KL, Gulack BC, Englum BR, et al.: Factors impacting survival in children with renal cell carcinoma. J Pediatr Surg 50 (6): 1014-8, 2015.[PUBMED Abstract]
- Selle B, Furtwängler R, Graf N, et al.: Population-based study of renal cell carcinoma in children in Germany, 1980-2005: more frequently localized tumors and underlying disorders compared with adult counterparts. Cancer 107 (12): 2906-14, 2006.[PUBMED Abstract]
- Cook A, Lorenzo AJ, Salle JL, et al.: Pediatric renal cell carcinoma: single institution 25-year case series and initial experience with partial nephrectomy. J Urol 175 (4): 1456-60; discussion 1460, 2006.[PUBMED Abstract]
- Fyfe G, Fisher RI, Rosenberg SA, et al.: Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 13 (3): 688-96, 1995.[PUBMED Abstract]
- Coppin C, Porzsolt F, Awa A, et al.: Immunotherapy for advanced renal cell cancer. Cochrane Database Syst Rev (1): CD001425, 2005.[PUBMED Abstract]
- De Pasquale MD, Pessolano R, Boldrini R, et al.: Continuing response to subsequent treatment lines with tyrosine kinase inhibitors in an adolescent with metastatic renal cell carcinoma. J Pediatr Hematol Oncol 33 (5): e176-9, 2011.[PUBMED Abstract]
- Chowdhury T, Prichard-Jones K, Sebire NJ, et al.: Persistent complete response after single-agent sunitinib treatment in a case of TFE translocation positive relapsed metastatic pediatric renal cell carcinoma. J Pediatr Hematol Oncol 35 (1): e1-3, 2013.[PUBMED Abstract]
- Wedekind MF, Ranalli M, Shah N: Clinical efficacy of cabozantinib in two pediatric patients with recurrent renal cell carcinoma. Pediatr Blood Cancer 64 (11): , 2017.[PUBMED Abstract]
-
von Hippel-Lindau (VHL) disease. VHL disease is an autosomal dominant condition in which blood vessels in the retina and cerebellum grow excessively.[
3
] The gene for VHL disease is located on chromosome 3p26 and is a tumor-suppressor gene, which is either mutated or deleted in patients with the syndrome.
- Rhabdoid Tumors of the Kidney
-
General Information About Rhabdoid Tumors of the Kidney
Rhabdoid tumors are extremely aggressive malignancies that generally occur in infants and young children. The most common locations are the kidney (termed malignant rhabdoid tumors) and the central nervous system (CNS) (atypical teratoid/rhabdoid tumor), although rhabdoid tumors can also arise in most soft tissue sites. (Refer to the PDQ summary on Childhood Central Nervous System Atypical Teratoid/Rhabdoid Tumor Treatment for information about the treatment of CNS disease.) Relapses occur early (median time from diagnosis, 8 months).[ 1 ][ 2 ]
A distinct clinical presentation that suggests a diagnosis of rhabdoid tumor of the kidney includes the following:[ 3 ]
- Fever.
- Hematuria.
- Young age (mean age, 11 months).
- Advanced tumor stage at presentation.
(Refer to the Clinical Features of Wilms Tumor and Diagnostic and Staging Evaluation for Wilms Tumor sections of this summary for more information about the clinical features and diagnostic evaluation of childhood kidney tumors.)
Approximately two-thirds of patients will present with advanced-stage disease. Bilateral cases have been reported.[ 1 ] Rhabdoid tumors of the kidney tend to metastasize to the lungs and the brain. As many as 10% to 15% of patients with rhabdoid tumors of the kidney also have CNS lesions.[ 4 ] The staging system used for rhabdoid tumor of the kidney is the same system used for Wilms tumor. (Refer to the Stage Information for Wilms Tumor section of this summary for more information.)
Histologically, the most distinctive features of rhabdoid tumors of the kidney are rather large cells with large vesicular nuclei, a prominent single nucleolus, and in some cells, the presence of globular eosinophilic cytoplasmic inclusions.
Genomics of Rhabdoid Tumors of the Kidney
Rhabdoid tumors in all anatomical locations have a common genetic abnormality—loss of function of the SMARCB1 (INI1/SNF5/BAF47) gene located at chromosome 22q11.2. The following text refers to rhabdoid tumors without regard to their primary site. SMARCB1 encodes a component of the SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeling complex that has an important role in controlling gene transcription.[ 5 ][ 6 ] Loss of function occurs by deletions that lead to loss of part or all of the SMARCB1 gene and by mutations that are commonly frameshift or nonsense mutations that lead to premature truncation of the SMARCB1 protein.[ 6 ][ 7 ] A small percentage of rhabdoid tumors are caused by alterations in SMARCA4, which is the primary ATPase in the SWI/SNF complex.[ 8 ][ 9 ] Exome sequencing of 35 cases of rhabdoid tumor identified a very low mutation rate, with no genes having recurring mutations other than SMARCB1, which appeared to contribute to tumorigenesis.[ 10 ]
Germline mutations of SMARCB1 have been documented in patients with one or more primary tumors of the brain and/or kidney, consistent with a genetic predisposition to the development of rhabdoid tumors.[ 11 ][ 12 ] Approximately one-third of patients with rhabdoid tumors have germline SMARCB1 alterations.[ 6 ][ 13 ] In most cases, the mutations are de novo and not inherited. The median age at diagnosis of children with rhabdoid tumors and a germline mutation or deletion is younger (6 months) than that of children with apparently sporadic disease (18 months).[ 14 ] Germline mosaicism has been suggested for several families with multiple affected siblings. It appears that patients with germline mutations may have the worst prognosis.[ 15 ][ 16 ] Germline mutations in SMARCA4 have also been reported in patients with rhabdoid tumors.[ 8 ][ 17 ]
Rhabdoid Tumor Predisposition Syndrome
Early-onset, multifocal disease and familial cases strongly support the possibility of a rhabdoid tumor predisposition syndrome. This has been confirmed by the presence of germline mutations of SMARCB1 in rare familial cases and in a subset of patients with apparently sporadic rhabdoid tumors. These cases have been labeled as rhabdoid tumor predisposition syndrome, type 1. Thirty-five patients (N = 100) with rhabdoid tumors of the brain, kidney, or soft tissues were found to have a germline SMARCB1 abnormality. These abnormalities included point and frameshift mutations, intragenic deletions and duplications, and larger deletions. Nine cases demonstrated parent-to-child transmission of a mutated copy of SMARCB1. In eight of the nine cases, one or more family members were also diagnosed with rhabdoid tumor or schwannoma; two of the eight families presented with multiple affected children, consistent with gonadal mosaicism.[ 6 ]
Two cases of inactivating mutations in the SMARCA4 gene have been found in three children from two unrelated families, establishing the phenotypically similar syndrome now known as rhabdoid tumor predisposition syndrome, type 2.[ 8 ][ 9 ] In these cases, SMARCA4 behaves as a classical tumor suppressor, with one deleterious mutation inherited in the germline and the other acquired in the tumor. Another report describes an autosomal dominant pattern of inheritance discovered through an exome sequencing project.[ 18 ]
Genetic Testing and Surveillance of Rhabdoid Tumors of the Kidney
Germline analysis is suggested for individuals of all ages with rhabdoid tumors. Genetic counseling is also part of the treatment plan, given the low-but-actual risk of familial recurrence. In cases of mutations, parental screening should be considered, although such screening carries a low probability of positivity. Prenatal diagnosis can be performed in situations in which a specific SMARCB1 mutation or deletion has been documented in the family.[ 6 ]
To date, there is little evidence regarding the effectiveness of surveillance for patients with rhabdoid tumor predisposition syndrome, type 1 caused by loss-of-function germline SMARCB1 mutations. However, because of the aggressive nature of the tumors with significant lethality and young age of onset in SMARCB1 carriers with truncating mutations, consensus recommendations have been developed. These recommendations were developed by a group of pediatric cancer genetic experts (including oncologists, radiologists, and geneticists). They have not been formally studied to confirm the benefit of monitoring patients with germline SMARCB1 mutations. Given the potential survival benefit of surgically resectable disease, it is postulated that early detection might improve overall survival (OS).[ 19 ][ 20 ][ 21 ]
Surveillance for patients with germline SMARCB1 mutations includes the following:
- Brain magnetic resonance imaging (MRI) every 3 months from birth (or diagnosis) until age 5 years.
- Abdominal ultrasonography with a focus on the kidneys are suggested every 3 months.
Prognosis and Prognostic Factors for Rhabdoid Tumors of the Kidney
Patients with rhabdoid tumors of the kidney continue to have a poor prognosis. In a review of 142 patients from the National Wilms Tumor Studies (NWTS) (NWTS-1, NWTS-2, NWTS-3, NWTS-4, and NWTS-5 [COG-Q9401/NCT00002611]), age and stage were identified as important prognostic factors:[ 4 ]
- Age at diagnosis. Infants younger than 6 months at diagnosis demonstrated a 4-year OS rate of 9%, whereas the OS rate in patients aged 2 years and older was 41% (highly significant).
- Stage of disease. Patients with stage I and stage II disease had an OS rate of 42%; higher stage was associated with a 16% OS rate.
- Presence of a CNS lesion. All but one patient with a CNS lesion (n = 32) died.
Treatment of Rhabdoid Tumor of the Kidney
Because of the relative rarity of this tumor, all patients with rhabdoid tumor of the kidney should be considered for entry into a clinical trial. Treatment planning by a multidisciplinary team of cancer specialists (pediatric surgeon or pediatric urologist, pediatric radiation oncologist, and pediatric oncologist) with experience treating renal tumors is required to determine and implement optimal treatment.
There is no standard treatment option for rhabdoid tumor of the kidney.[ 22 ]
The following results have been observed in studies of rhabdoid tumor of the kidney:
- On the basis of a retrospective comparison of tumor response to preoperative treatment with vincristine/dactinomycin versus vincristine/dactinomycin/doxorubicin, doxorubicin is considered an active drug in malignant rhabdoid tumor of the kidney.[ 23 ][Level of evidence: 3iiiDiv]
- The NWTS-5 trial closed the arm for rhabdoid tumor treatment with cyclophosphamide, etoposide, and carboplatin because poor outcome was observed. Combinations of etoposide and cisplatin; etoposide and ifosfamide; and ifosfamide, carboplatin, and etoposide (ICE chemotherapy) have been used.[ 24 ][ 25 ]
- Treatment with high-dose alkylator therapy followed by consolidation with high-dose chemotherapy and, in some cases, autologous stem cell transplant after achieving a radiographic remission has resulted in some long-term survival (5 of 13 patients). None of the patients with unresectable primary tumors survived in this small series (N = 21).[ 26 ]
- A retrospective analysis of 58 patients with malignant rhabdoid tumor of the kidney from the International Society of Pediatric Oncology (SIOP), Gesellschaft für Pädiatrische Onkologie und Hämatologie (GPOH), and European Rhabdoid Tumor Registry was performed.[
27
]
- For the entire group, the 2-year event-free survival (EFS) rate was 37%, and the OS rate was 38%.
- Patients with multifocal involvement (n = 12) had significantly inferior survival than did patients with pulmonary or mediastinal metastases or local disease.
- Patients who underwent upfront chemotherapy had a lower, but not statistically significant, 2-year EFS than did patients who underwent immediate surgical resection.
- Younger age (<12 months) and local stage III disease were associated with significantly inferior survival than were stage I and stage II disease.
- No difference was seen in 2-year EFS for patients without progression within 90 days consolidated by high-dose stem cell transplantation (SCT) (n = 10) compared with patients without consolidation by SCT (n = 21).
Treatment Options Under Clinical Evaluation for Rhabdoid Tumors of the Kidney
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
-
APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI–Children's Oncology Group Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified using a next-generation sequencing targeted assay of more than 4,000 different mutations across more than 160 genes in refractory and recurrent solid tumors. Children and adolescents aged 1 to 21 years are eligible for the trial.
Tumor tissue from progressive or recurrent disease must be available for molecular characterization. Patients with tumors that have molecular variants addressed by treatment arms included in the trial will be offered treatment on Pediatric MATCH. Additional information can be obtained on the NCI website and ClinicalTrials.gov website.
- NCT02601937 (A Phase 1 Study of the EZH2 Inhibitor Tazemetostat in Pediatric Subjects With Relapsed or Refractory INI1-Negative Tumors or Synovial Sarcoma): Patients with INI1-negative tumors are eligible for targeted treatment with an EZH2 inhibitor. This is a phase I, open-label, dose-escalation, and dose-expansion study with a twice-daily oral dose of tazemetostat.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
参考文献- van den Heuvel-Eibrink MM, van Tinteren H, Rehorst H, et al.: Malignant rhabdoid tumours of the kidney (MRTKs), registered on recent SIOP protocols from 1993 to 2005: a report of the SIOP renal tumour study group. Pediatr Blood Cancer 56 (5): 733-7, 2011.[PUBMED Abstract]
- Reinhard H, Reinert J, Beier R, et al.: Rhabdoid tumors in children: prognostic factors in 70 patients diagnosed in Germany. Oncol Rep 19 (3): 819-23, 2008.[PUBMED Abstract]
- Amar AM, Tomlinson G, Green DM, et al.: Clinical presentation of rhabdoid tumors of the kidney. J Pediatr Hematol Oncol 23 (2): 105-8, 2001.[PUBMED Abstract]
- Tomlinson GE, Breslow NE, Dome J, et al.: Rhabdoid tumor of the kidney in the National Wilms' Tumor Study: age at diagnosis as a prognostic factor. J Clin Oncol 23 (30): 7641-5, 2005.[PUBMED Abstract]
- Imbalzano AN, Jones SN: Snf5 tumor suppressor couples chromatin remodeling, checkpoint control, and chromosomal stability. Cancer Cell 7 (4): 294-5, 2005.[PUBMED Abstract]
- Eaton KW, Tooke LS, Wainwright LM, et al.: Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer 56 (1): 7-15, 2011.[PUBMED Abstract]
- Versteege I, Sévenet N, Lange J, et al.: Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 394 (6689): 203-6, 1998.[PUBMED Abstract]
- Schneppenheim R, Frühwald MC, Gesk S, et al.: Germline nonsense mutation and somatic inactivation of SMARCA4/BRG1 in a family with rhabdoid tumor predisposition syndrome. Am J Hum Genet 86 (2): 279-84, 2010.[PUBMED Abstract]
- Hasselblatt M, Gesk S, Oyen F, et al.: Nonsense mutation and inactivation of SMARCA4 (BRG1) in an atypical teratoid/rhabdoid tumor showing retained SMARCB1 (INI1) expression. Am J Surg Pathol 35 (6): 933-5, 2011.[PUBMED Abstract]
- Lee RS, Stewart C, Carter SL, et al.: A remarkably simple genome underlies highly malignant pediatric rhabdoid cancers. J Clin Invest 122 (8): 2983-8, 2012.[PUBMED Abstract]
- Biegel JA, Zhou JY, Rorke LB, et al.: Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res 59 (1): 74-9, 1999.[PUBMED Abstract]
- Biegel JA: Molecular genetics of atypical teratoid/rhabdoid tumor. Neurosurg Focus 20 (1): E11, 2006.[PUBMED Abstract]
- Bourdeaut F, Lequin D, Brugières L, et al.: Frequent hSNF5/INI1 germline mutations in patients with rhabdoid tumor. Clin Cancer Res 17 (1): 31-8, 2011.[PUBMED Abstract]
- Geller JI, Roth JJ, Biegel JA: Biology and Treatment of Rhabdoid Tumor. Crit Rev Oncog 20 (3-4): 199-216, 2015.[PUBMED Abstract]
- Janson K, Nedzi LA, David O, et al.: Predisposition to atypical teratoid/rhabdoid tumor due to an inherited INI1 mutation. Pediatr Blood Cancer 47 (3): 279-84, 2006.[PUBMED Abstract]
- Sévenet N, Sheridan E, Amram D, et al.: Constitutional mutations of the hSNF5/INI1 gene predispose to a variety of cancers. Am J Hum Genet 65 (5): 1342-8, 1999.[PUBMED Abstract]
- Hasselblatt M, Nagel I, Oyen F, et al.: SMARCA4-mutated atypical teratoid/rhabdoid tumors are associated with inherited germline alterations and poor prognosis. Acta Neuropathol 128 (3): 453-6, 2014.[PUBMED Abstract]
- Witkowski L, Lalonde E, Zhang J, et al.: Familial rhabdoid tumour 'avant la lettre'--from pathology review to exome sequencing and back again. J Pathol 231 (1): 35-43, 2013.[PUBMED Abstract]
- Teplick A, Kowalski M, Biegel JA, et al.: Educational paper: screening in cancer predisposition syndromes: guidelines for the general pediatrician. Eur J Pediatr 170 (3): 285-94, 2011.[PUBMED Abstract]
- Mitchell SG, Pencheva B, Porter CC: Germline Genetics and Childhood Cancer: Emerging Cancer Predisposition Syndromes and Psychosocial Impacts. Curr Oncol Rep 21 (10): 85, 2019.[PUBMED Abstract]
- Foulkes WD, Kamihara J, Evans DGR, et al.: Cancer Surveillance in Gorlin Syndrome and Rhabdoid Tumor Predisposition Syndrome. Clin Cancer Res 23 (12): e62-e67, 2017.[PUBMED Abstract]
- Ahmed HU, Arya M, Levitt G, et al.: Part II: Treatment of primary malignant non-Wilms' renal tumours in children. Lancet Oncol 8 (9): 842-8, 2007.[PUBMED Abstract]
- Furtwängler R, Nourkami-Tutdibi N, Leuschner I, et al.: Malignant rhabdoid tumor of the kidney: significantly improved response to pre-operative treatment intensified with doxorubicin. Cancer Genet 207 (9): 434-6, 2014.[PUBMED Abstract]
- Waldron PE, Rodgers BM, Kelly MD, et al.: Successful treatment of a patient with stage IV rhabdoid tumor of the kidney: case report and review. J Pediatr Hematol Oncol 21 (1): 53-7, 1999 Jan-Feb.[PUBMED Abstract]
- Wagner L, Hill DA, Fuller C, et al.: Treatment of metastatic rhabdoid tumor of the kidney. J Pediatr Hematol Oncol 24 (5): 385-8, 2002 Jun-Jul.[PUBMED Abstract]
- Venkatramani R, Shoureshi P, Malvar J, et al.: High dose alkylator therapy for extracranial malignant rhabdoid tumors in children. Pediatr Blood Cancer 61 (8): 1357-61, 2014.[PUBMED Abstract]
- Furtwängler R, Kager L, Melchior P, et al.: High-dose treatment for malignant rhabdoid tumor of the kidney: No evidence for improved survival-The Gesellschaft für Pädiatrische Onkologie und Hämatologie (GPOH) experience. Pediatr Blood Cancer 65 (1): , 2018.[PUBMED Abstract]
- Clear Cell Sarcoma of the Kidney
-
General Information About Clear Cell Sarcoma of the Kidney
Clear cell sarcoma of the kidney is not a Wilms tumor variant, but it is an important primary renal tumor associated with a higher rate of relapse and death than is favorable histology (FH) Wilms tumor.[ 1 ] The classic pattern of clear cell sarcoma of the kidney is defined by nests or cords of cells separated by regularly spaced fibrovascular septa. In addition to pulmonary metastases, clear cell sarcoma also spreads to bone, brain, and soft tissue.[ 1 ] (Refer to the Clinical Features of Wilms Tumor and Diagnostic and Staging Evaluation for Wilms Tumor sections of this summary for more information about the clinical features and diagnostic evaluation of childhood kidney tumors.)
Younger age and stage IV disease have been identified as adverse prognostic factors for event-free survival (EFS).[ 2 ]
Historically, relapses have occurred in long intervals after the completion of chemotherapy (up to 14 years); however, with current therapy, relapses after 3 years are uncommon.[ 3 ] The brain is a frequent site of recurrent disease, suggesting that it is a sanctuary site for cells that are protected from the intensive chemotherapy that patients currently receive.[ 2 ][ 3 ][ 4 ][ 5 ] An awareness of the clinical signs of recurrent disease in the brain is important during regular follow-up. There are no standard recommendations for the frequency of brain imaging during follow-up.
Genomics of Clear Cell Sarcoma of the Kidney
Clear cell sarcoma of the kidney is an uncommon renal tumor that comprises approximately 5% of all primary renal malignancies in children, accounts for approximately 20 new cases per year in the United States, and is observed most often before age 3 years.[ 1 ] The molecular background of clear cell sarcoma of the kidney is poorly understood because of its rarity and lack of experimental models.
Several biological features of clear cell sarcoma of the kidney have been described, including the following:
- Internal tandem duplications in exon 15 of the BCOR gene (BCL6 corepressor) have been reported in 90% of cases of clear cell sarcoma of the kidney, with a smaller subset harboring YWHAE-NUTM2B/E or BCOR-CCNB3 gene fusions.[ 6 ][ 7 ][ 8 ][ 9 ][ 10 ][ 11 ] All of these genetic abnormalities result in a transcriptional signature characterized by high BCOR mRNA expression.[ 12 ]
- Diffuse strong immunoreactivity for BCOR is highly sensitive and specific for the diagnosis of clear cell sarcoma of the kidney. In a series of 79 neoplasms—including Wilms tumors, congenital mesoblastic nephromas, clear cell sarcoma of the kidney, metanephric stromal tumors, rhabdoid tumors of the kidney, renal primitive neuroectodermal tumor (PNET), and sclerosing epithelioid fibrosarcomas—all of the clear cell sarcomas of the kidney samples that were tested demonstrated diffuse, strong nuclear labeling for BCOR. Most of the other pediatric renal neoplasms were completely negative for BCOR.[ 13 ]
Treatment of Clear Cell Sarcoma of the Kidney
Because of the relative rarity of this tumor, all patients with clear cell sarcoma of the kidney should be considered for entry into a clinical trial. Treatment planning by a multidisciplinary team of cancer specialists (pediatric surgeon or pediatric urologist, pediatric radiation oncologist, and pediatric oncologist) with experience treating renal tumors is required to determine and implement optimal treatment.
The approach for treating clear cell sarcoma of the kidney is different from the approach for treating Wilms tumor because the overall survival (OS) of children with clear cell sarcoma of the kidney remains lower than that for patients with FH Wilms tumor. All patients undergo postoperative radiation to the tumor bed and receive doxorubicin as part of their chemotherapy regimen.
The standard treatment option for clear cell sarcoma of the kidney is the following:
Surgery, chemotherapy, and radiation therapy
Evidence (surgery, chemotherapy, and radiation therapy):
- In the National Wilms Tumor Study (NWTS)-3 trial (NWTS-3), the addition of doxorubicin to the combination of vincristine, dactinomycin, and radiation therapy resulted in an improvement in disease-free survival for patients with clear cell sarcoma of the kidney.[ 1 ]
- The NWTS-4 trial used regimen DD-4A, which consisted of vincristine, dactinomycin, and doxorubicin for 15 months, and radiation therapy.[
14
]
- NWTS-4 reported that patients who were treated with vincristine, doxorubicin, and dactinomycin for 15 months had an improved relapse-free survival rate compared with patients who were treated for 6 months (88% vs. 61% at 8 years).
- In the NWTS-5 (COG-Q9401/NCT00002611) trial, children with stages
I to IV clear cell sarcoma of the kidney were treated with a new
chemotherapeutic regimen combining vincristine, doxorubicin, cyclophosphamide,
and etoposide in an attempt to further improve the survival of these high-risk
groups. All patients received radiation therapy to the tumor bed.[
3
]
- With this treatment, the 5-year EFS rate was 79%, and the OS rate was 90%.
- Stage I patients had 5-year EFS and OS rates of 100%.
- Stage II patients had a 5-year EFS rate of 88% and a 5-year OS rate of 98%.
- Stage III patients had a 5-year EFS rate of 73% and a 5-year OS rate of 89%.
- Stage IV patients had a 5-year EFS rate of 29% and a 5-year OS rate of 36%.
- A review of patients with stage I clear cell sarcoma of the kidney treated on the NWTS-1, NWTS-2, NWTS-3, NWTS-4, and NWTS-5 trials showed an excellent OS rate of 100% with a wide variety of chemotherapy and radiation therapy regimens.[ 15 ]
(Refer to the Treatment of Recurrent Clear Cell Sarcoma of the Kidney section of this summary for information about recurrent disease.)
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
参考文献- Argani P, Perlman EJ, Breslow NE, et al.: Clear cell sarcoma of the kidney: a review of 351 cases from the National Wilms Tumor Study Group Pathology Center. Am J Surg Pathol 24 (1): 4-18, 2000.[PUBMED Abstract]
- Furtwängler R, Gooskens SL, van Tinteren H, et al.: Clear cell sarcomas of the kidney registered on International Society of Pediatric Oncology (SIOP) 93-01 and SIOP 2001 protocols: a report of the SIOP Renal Tumour Study Group. Eur J Cancer 49 (16): 3497-506, 2013.[PUBMED Abstract]
- Seibel NL, Chi YY, Perlman EJ, et al.: Impact of cyclophosphamide and etoposide on outcome of clear cell sarcoma of the kidney treated on the National Wilms Tumor Study-5 (NWTS-5). Pediatr Blood Cancer 66 (1): e27450, 2019.[PUBMED Abstract]
- Radulescu VC, Gerrard M, Moertel C, et al.: Treatment of recurrent clear cell sarcoma of the kidney with brain metastasis. Pediatr Blood Cancer 50 (2): 246-9, 2008.[PUBMED Abstract]
- Gooskens SL, Furtwängler R, Spreafico F, et al.: Treatment and outcome of patients with relapsed clear cell sarcoma of the kidney: a combined SIOP and AIEOP study. Br J Cancer 111 (2): 227-33, 2014.[PUBMED Abstract]
- Ueno-Yokohata H, Okita H, Nakasato K, et al.: Consistent in-frame internal tandem duplications of BCOR characterize clear cell sarcoma of the kidney. Nat Genet 47 (8): 861-3, 2015.[PUBMED Abstract]
- Argani P, Kao YC, Zhang L, et al.: Primary Renal Sarcomas With BCOR-CCNB3 Gene Fusion: A Report of 2 Cases Showing Histologic Overlap With Clear Cell Sarcoma of Kidney, Suggesting Further Link Between BCOR-related Sarcomas of the Kidney and Soft Tissues. Am J Surg Pathol 41 (12): 1702-1712, 2017.[PUBMED Abstract]
- Karlsson J, Valind A, Gisselsson D: BCOR internal tandem duplication and YWHAE-NUTM2B/E fusion are mutually exclusive events in clear cell sarcoma of the kidney. Genes Chromosomes Cancer 55 (2): 120-3, 2016.[PUBMED Abstract]
- Astolfi A, Melchionda F, Perotti D, et al.: Whole transcriptome sequencing identifies BCOR internal tandem duplication as a common feature of clear cell sarcoma of the kidney. Oncotarget 6 (38): 40934-9, 2015.[PUBMED Abstract]
- Roy A, Kumar V, Zorman B, et al.: Recurrent internal tandem duplications of BCOR in clear cell sarcoma of the kidney. Nat Commun 6: 8891, 2015.[PUBMED Abstract]
- Wong MK, Ng CCY, Kuick CH, et al.: Clear cell sarcomas of the kidney are characterised by BCOR gene abnormalities, including exon 15 internal tandem duplications and BCOR-CCNB3 gene fusion. Histopathology 72 (2): 320-329, 2018.[PUBMED Abstract]
- Kao YC, Sung YS, Zhang L, et al.: Recurrent BCOR Internal Tandem Duplication and YWHAE-NUTM2B Fusions in Soft Tissue Undifferentiated Round Cell Sarcoma of Infancy: Overlapping Genetic Features With Clear Cell Sarcoma of Kidney. Am J Surg Pathol 40 (8): 1009-20, 2016.[PUBMED Abstract]
- Argani P, Pawel B, Szabo S, et al.: Diffuse Strong BCOR Immunoreactivity Is a Sensitive and Specific Marker for Clear Cell Sarcoma of the Kidney (CCSK) in Pediatric Renal Neoplasia. Am J Surg Pathol 42 (8): 1128-1131, 2018.[PUBMED Abstract]
- Seibel NL, Li S, Breslow NE, et al.: Effect of duration of treatment on treatment outcome for patients with clear-cell sarcoma of the kidney: a report from the National Wilms' Tumor Study Group. J Clin Oncol 22 (3): 468-73, 2004.[PUBMED Abstract]
- Kalapurakal JA, Perlman EJ, Seibel NL, et al.: Outcomes of patients with revised stage I clear cell sarcoma of kidney treated in National Wilms Tumor Studies 1-5. Int J Radiat Oncol Biol Phys 85 (2): 428-31, 2013.[PUBMED Abstract]
- Congenital Mesoblastic Nephroma
-
General Information About Congenital Mesoblastic Nephroma
Mesoblastic nephroma comprises about 5% of childhood kidney tumors, and more than 90% of cases appear within the first year of life. More than 15% of the cases are detected prenatally.[ 1 ] It is the most common kidney tumor found in infants younger than 6 months.[ 2 ] The median age of diagnosis is 1 to 2 months. Twice as many males as females are diagnosed. The diagnosis should be questioned when applied to individuals older than 2 years.[ 1 ]
When patients are diagnosed in the first 7 months of life, the 5-year event-free survival rate is 94%, and the overall survival (OS) rate is 96%.[ 3 ] In a report from the United Kingdom of 50 children with mesoblastic nephroma studied on clinical trials and 80 cases from the national registry in the same time period, there were no deaths.[ 1 ] However, in a comprehensive review of the literature, 12 deaths were reported; of these 12 deaths, 7 were from surgical complications in infants.[ 4 ][Level of evidence: 3iiiDii]
Grossly, mesoblastic nephromas appear as solitary, unilateral masses indistinguishable from nephroblastoma. Microscopically, they consist of spindled mesenchymal cells. Mesoblastic nephroma can be divided into the following three histologic subtypes:
A frequent genetic alteration is the translocation t(12;15)(q13;q25), resulting in a fusion of the ETV6 and NTRK3 genes on 15p15 that occurs almost exclusively in the cellular type of mesoblastic nephroma. In a cohort of 79 mesoblastic nephromas analyzed for the translocation, all classical (n = 38) and mixed (n = 12) mesoblastic nephromas were translocation negative.[ 8 ] The same translocation was initially described in infantile fibrosarcoma, and besides the similar morphologic appearance, cases of cellular mesoblastic nephroma and infantile fibrosarcoma share other genetic changes such as gains of chromosome 11.[ 9 ]
The risk of recurrence for patients with mesoblastic nephroma is closely associated with the presence of a cellular subtype and with stage III disease.[ 5 ] In an International Society of Pediatric Oncology (SIOP) series of 79 patients with congenital mesoblastic nephromas, patients within the cellular subgroup who had translocation-positive tumors had a significantly superior relapse-free survival (RFS) rate when compared with patients who did not have the gene fusion (100% vs. 73%, respectively).[ 8 ]
(Refer to the Clinical Features of Wilms Tumor and Diagnostic and Staging Evaluation for Wilms Tumor sections of this summary for more information about the clinical features and diagnostic evaluation of childhood kidney tumors.)
Treatment of Congenital Mesoblastic Nephroma
The OS of patients with congenital mesoblastic nephroma is excellent; however, reported causes of death in about one-half of the cases are treatment related, and most of these patients were very young (median age, <1 year).[ 4 ] This underscores the special attention that infants with renal tumors require, with respect to timing and type of treatment and the importance of a dedicated expert pediatric oncology setting.
Standard treatment options for stages I and II (80% of patients) and stage III (classic and mixed subtypes) congenital mesoblastic nephroma include the following:
Treatment options for stage III (cellular subtype) congenital mesoblastic nephroma include the following:
- Nephrectomy.
- Chemotherapy.
Nephrectomy
Evidence (nephrectomy):
- In a SIOP/Gesellschaft für Pädiatrische Hämatologie und Onkologie (GPOH) nephroblastoma study, 111 patients with congenital mesoblastic nephromas demonstrated a 5-year RFS rate of 93.2% and a 5-year OS rate of 96.8%.[
8
]
- Sixty-seven patients had classical congenital mesoblastic nephromas (60%), 29 patients had the cellular subtype (26%), and 15 patients had the mixed subtype (14%). The 5-year RFS rate was significantly superior for the classical type (98%) compared with the cellular type (89%, P = .039) or mixed type (80%, P = .002). There was no significant difference in OS by tumor types.
- Tumor stage consisted of stage I (35%), stage II (50%), and stage III (15%).
- Ninety-one patients were treated with surgery alone, and 19 patients were treated with chemotherapy in addition to surgery (12 preoperatively and 11 postoperatively).
- Seven patients relapsed (five local and two combined) and three patients died because of local relapse (one of each histologic type).
- Tissue was available for translocation analysis from 79 of the 111 tumors. Within the group of cellular congenital mesoblastic nephromas, patients who had translocation-positive tumors had a significantly improved RFS compared with the patients who had translocation-negative tumors (5-year RFS rate, 100% vs. 73%).
Adjuvant chemotherapy
Adjuvant chemotherapy has been recommended for patients with stage III cellular subtype mesoblastic nephromas who are aged 3 months or older at diagnosis.[ 5 ] In a study of stage III cellular type congenital mesoblastic nephroma, 7 of 12 patients who were treated with surgery only suffered from a relapse, while 4 of 14 patients who were treated with adjuvant chemotherapy (primarily dactinomycin/vincristine and sometimes doxorubicin) developed a relapse.[ 1 ][ 5 ][ 10 ] Cyclophosphamide and ifosfamide have been combined with these agents and have shown activity.[ 11 ]
Infants younger than 2 months with incompletely resected, stage III disease may not need chemotherapy.[ 1 ]
(Refer to the Treatment of Recurrent Congenital Mesoblastic Nephroma section of this summary for information about recurrent disease.)
Treatment options under clinical evaluation for congenital mesoblastic nephroma
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
- ADVL1823 (NCT03834961) (Larotrectinib in Treating Patients With Previously Untreated TRK Fusion Solid Tumors and TRK Fusion Relapsed Acute Leukemia): This is a phase II trial to study how well larotrectinib works in treating patients with previously untreated TRK fusion solid tumors and TRK fusion acute leukemia that has come back. Larotrectinib is a highly selective oral small molecule inhibitor of the TRK family of tyrosine kinases (TRKA, TRKB, and TRKC), which are encoded by the NTRK genes. Larotrectinib will be administered twice daily on a continuous dosing schedule.
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
参考文献- England RJ, Haider N, Vujanic GM, et al.: Mesoblastic nephroma: a report of the United Kingdom Children's Cancer and Leukaemia Group (CCLG). Pediatr Blood Cancer 56 (5): 744-8, 2011.[PUBMED Abstract]
- Jehangir S, Kurian JJ, Selvarajah D, et al.: Recurrent and metastatic congenital mesoblastic nephroma: where does the evidence stand? Pediatr Surg Int 33 (11): 1183-1188, 2017.[PUBMED Abstract]
- van den Heuvel-Eibrink MM, Grundy P, Graf N, et al.: Characteristics and survival of 750 children diagnosed with a renal tumor in the first seven months of life: A collaborative study by the SIOP/GPOH/SFOP, NWTSG, and UKCCSG Wilms tumor study groups. Pediatr Blood Cancer 50 (6): 1130-4, 2008.[PUBMED Abstract]
- Gooskens SL, Houwing ME, Vujanic GM, et al.: Congenital mesoblastic nephroma 50 years after its recognition: A narrative review. Pediatr Blood Cancer 64 (7): , 2017.[PUBMED Abstract]
- Furtwaengler R, Reinhard H, Leuschner I, et al.: Mesoblastic nephroma--a report from the Gesellschaft fur Pädiatrische Onkologie und Hämatologie (GPOH). Cancer 106 (10): 2275-83, 2006.[PUBMED Abstract]
- El Demellawy D, Cundiff CA, Nasr A, et al.: Congenital mesoblastic nephroma: a study of 19 cases using immunohistochemistry and ETV6-NTRK3 fusion gene rearrangement. Pathology 48 (1): 47-50, 2016.[PUBMED Abstract]
- Argani P, Ladanyi M: Recent advances in pediatric renal neoplasia. Adv Anat Pathol 10 (5): 243-60, 2003.[PUBMED Abstract]
- Vokuhl C, Nourkami-Tutdibi N, Furtwängler R, et al.: ETV6-NTRK3 in congenital mesoblastic nephroma: A report of the SIOP/GPOH nephroblastoma study. Pediatr Blood Cancer 65 (4): , 2018.[PUBMED Abstract]
- Knezevich SR, Garnett MJ, Pysher TJ, et al.: ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res 58 (22): 5046-8, 1998.[PUBMED Abstract]
- Bayindir P, Guillerman RP, Hicks MJ, et al.: Cellular mesoblastic nephroma (infantile renal fibrosarcoma): institutional review of the clinical, diagnostic imaging, and pathologic features of a distinctive neoplasm of infancy. Pediatr Radiol 39 (10): 1066-74, 2009.[PUBMED Abstract]
- McCahon E, Sorensen PH, Davis JH, et al.: Non-resectable congenital tumors with the ETV6-NTRK3 gene fusion are highly responsive to chemotherapy. Med Pediatr Oncol 40 (5): 288-92, 2003.[PUBMED Abstract]
- Ewing Sarcoma of the Kidney
-
General Information About Ewing Sarcoma of the Kidney
Ewing sarcoma (previously known as neuroepithelial tumor) of the kidney is extremely rare and demonstrates a unique proclivity for young adults. It is a highly aggressive neoplasm, more often presenting with large tumors and penetration of the renal capsule, extension into the renal vein, and in 40% of cases, evidence of metastases.[ 1 ][ 2 ][ 3 ]
Ewing sarcoma of the kidney is characterized by CD99 (MIC-2) positivity and the detection of EWS/FLI-1 fusion transcripts. In Ewing sarcoma of the kidney, focal, atypical histologic features have been seen, including clear cell sarcoma, rhabdoid tumor, malignant peripheral nerve sheath tumors, and paraganglioma.[ 1 ][ 4 ] (Refer to the PDQ summary on Ewing Sarcoma Treatment for more information.)
Treatment of Ewing Sarcoma of the Kidney
There is no standard treatment option for Ewing sarcoma of the kidney. However, treatment with chemotherapy and radiation therapy and an aggressive surgical approach seem to be associated with a better outcome than previously reported.[ 2 ] Consideration should also be given to substituting cyclophosphamide for ifosfamide in patients after they have undergone a nephrectomy. [ 2 ][ 3 ]
Treatment according to Ewing sarcoma protocols should be considered.[ 1 ]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
参考文献- Parham DM, Roloson GJ, Feely M, et al.: Primary malignant neuroepithelial tumors of the kidney: a clinicopathologic analysis of 146 adult and pediatric cases from the National Wilms' Tumor Study Group Pathology Center. Am J Surg Pathol 25 (2): 133-46, 2001.[PUBMED Abstract]
- Tagarelli A, Spreafico F, Ferrari A, et al.: Primary renal soft tissue sarcoma in children. Urology 80 (3): 698-702, 2012.[PUBMED Abstract]
- Rowe RG, Thomas DG, Schuetze SM, et al.: Ewing sarcoma of the kidney: case series and literature review of an often overlooked entity in the diagnosis of primary renal tumors. Urology 81 (2): 347-53, 2013.[PUBMED Abstract]
- Ellison DA, Parham DM, Bridge J, et al.: Immunohistochemistry of primary malignant neuroepithelial tumors of the kidney: a potential source of confusion? A study of 30 cases from the National Wilms Tumor Study Pathology Center. Hum Pathol 38 (2): 205-11, 2007.[PUBMED Abstract]
- Primary Renal Myoepithelial Carcinoma
-
General Information About Primary Renal Myoepithelial Carcinoma
Myoepithelial carcinomas are aggressive malignancies primarily affecting soft tissues with occasional visceral origin. Approximately 20% of all reported cases have been described in children and are associated with a particularly unfavorable outcome, frequent development of metastases, and short overall survival.[ 1 ]
Two cases of primary renal myoepithelial carcinoma have occurred in children, and both cases had a translocation involving EWSR1 and the novel fusion partner KLF15, a transcription factor uniquely functioning within the kidney. Helpful features to establish the diagnosis include coexpression of cytokeratins, S-100, and smooth muscle markers, and the documentation of EWSR1 rearrangements.[ 2 ]
Treatment of Primary Renal Myoepithelial Carcinoma
Although no standard therapy has been established, surgical resection of the primary tumor and pulmonary nodules (if present) has been used in addition to chemotherapy and radiation therapy.[ 2 ]
参考文献- Gleason BC, Fletcher CD: Myoepithelial carcinoma of soft tissue in children: an aggressive neoplasm analyzed in a series of 29 cases. Am J Surg Pathol 31 (12): 1813-24, 2007.[PUBMED Abstract]
- Cajaiba MM, Jennings LJ, Rohan SM, et al.: Expanding the Spectrum of Renal Tumors in Children: Primary Renal Myoepithelial Carcinomas With a Novel EWSR1-KLF15 Fusion. Am J Surg Pathol 40 (3): 386-94, 2016.[PUBMED Abstract]
- Cystic Partially Differentiated Nephroblastoma
-
General Information About Cystic Partially Differentiated Nephroblastoma
Cystic partially differentiated nephroblastoma is a rare cystic variant of Wilms tumor (1%), with unique pathologic and clinical characteristics. It is composed entirely of cysts, and their thin septa are the only solid portion of the tumor. The septa contain blastemal cells in any amount with or without embryonal stromal or epithelial cell type. Several pathologic features distinguish this neoplasm from standard Wilms tumor. DICER1 mutations have not been reported in cystic partially differentiated nephroblastoma, which supports a distinction between multilocular cystic nephromas and cystic partially differentiated nephroblastoma.[ 1 ]
Recurrence has been reported after tumor spillage during surgery.[ 2 ][Level of evidence: 3iiiA]
(Refer to the Clinical Features of Wilms Tumor and Diagnostic and Staging Evaluation for Wilms Tumor sections of this summary for more information about the clinical features and diagnostic evaluation of childhood kidney tumors.)
Treatment of Cystic Partially Differentiated Nephroblastoma
Standard treatment options for cystic partially differentiated nephroblastoma include the following:
参考文献- Cajaiba MM, Khanna G, Smith EA, et al.: Pediatric cystic nephromas: distinctive features and frequent DICER1 mutations. Hum Pathol 48: 81-7, 2016.[PUBMED Abstract]
- Baker JM, Viero S, Kim PC, et al.: Stage III cystic partially differentiated nephroblastoma recurring after nephrectomy and chemotherapy. Pediatr Blood Cancer 50 (1): 129-31, 2008.[PUBMED Abstract]
- Blakely ML, Shamberger RC, Norkool P, et al.: Outcome of children with cystic partially differentiated nephroblastoma treated with or without chemotherapy. J Pediatr Surg 38 (6): 897-900, 2003.[PUBMED Abstract]
- Multilocular Cystic Nephroma
-
General Information About Multilocular Cystic Nephroma
Multilocular cystic nephromas are uncommon benign lesions consisting of cysts lined by renal epithelium. They are characterized by a bimodal age distribution, affecting either infants/young children or adult females. These lesions can occur bilaterally, and a familial pattern has been reported.
Multilocular cystic nephroma has been associated with pleuropulmonary blastoma and the DICER1 mutation. Anaplastic sarcoma of the kidney has also been associated with the DICER1 mutation.[ 1 ] This is in contrast to adult cystic nephromas, which lack DICER1 mutations, and supports the difference between adult and pediatric cases. Genetic counseling, DICER1 mutation testing, and screening for lung lesions of a solid or cystic nature should be considered.[ 2 ][ 3 ][ 4 ][ 5 ]
(Refer to the Clinical Features of Wilms Tumor and Diagnostic and Staging Evaluation for Wilms Tumor sections of this summary for more information about the clinical features and diagnostic evaluation of childhood kidney tumors.)
Treatment of Multilocular Cystic Nephroma
The standard treatment option for multilocular cystic nephroma is surgery.
参考文献- Wu MK, Goudie C, Druker H, et al.: Evolution of Renal Cysts to Anaplastic Sarcoma of Kidney in a Child With DICER1 Syndrome. Pediatr Blood Cancer 63 (7): 1272-5, 2016.[PUBMED Abstract]
- Dehner LP, Messinger YH, Schultz KA, et al.: Pleuropulmonary Blastoma: Evolution of an Entity as an Entry into a Familial Tumor Predisposition Syndrome. Pediatr Dev Pathol 18 (6): 504-11, 2015 Nov-Dec.[PUBMED Abstract]
- Doros LA, Rossi CT, Yang J, et al.: DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Mod Pathol 27 (9): 1267-80, 2014.[PUBMED Abstract]
- Cajaiba MM, Khanna G, Smith EA, et al.: Pediatric cystic nephromas: distinctive features and frequent DICER1 mutations. Hum Pathol 48: 81-7, 2016.[PUBMED Abstract]
- Li Y, Pawel BR, Hill DA, et al.: Pediatric Cystic Nephroma Is Morphologically, Immunohistochemically, and Genetically Distinct From Adult Cystic Nephroma. Am J Surg Pathol 41 (4): 472-481, 2017.[PUBMED Abstract]
- Primary Renal Synovial Sarcoma
-
General Information About Primary Renal Synovial Sarcoma
Primary renal synovial sarcoma is a subset of embryonal sarcoma of the kidney and is characterized by the t(x;18)(p11;q11) SS18-SSX translocation. It is similar in histology to the monophasic spindle cell synovial sarcoma and is considered an aggressive tumor with adverse patient outcomes in more than 50% of cases (n = 16).[ 1 ] A second alternative gene fusion variant, SS18-NEDD4, has been identified in a primary renal synovial sarcoma.[ 2 ] Primary renal synovial sarcoma contains cystic structures derived from dilated, trapped renal tubules. Primary renal synovial sarcoma occurs more often in young adults.
(Refer to the Clinical Features of Wilms Tumor and Diagnostic and Staging Evaluation for Wilms Tumor sections of this summary for more information about the clinical features and diagnostic evaluation of childhood kidney tumors.)
Treatment of Primary Renal Synovial Sarcoma
The standard treatment option for primary renal synovial sarcoma is chemotherapy. The chemotherapy regimens used for primary renal synovial sarcoma differ from those traditionally used for Wilms tumor.[ 3 ]
参考文献- Schoolmeester JK, Cheville JC, Folpe AL: Synovial sarcoma of the kidney: a clinicopathologic, immunohistochemical, and molecular genetic study of 16 cases. Am J Surg Pathol 38 (1): 60-5, 2014.[PUBMED Abstract]
- Argani P, Zhang L, Sung YS, et al.: Novel SS18-NEDD4 gene fusion in a primary renal synovial sarcoma. Genes Chromosomes Cancer 59 (3): 203-208, 2020.[PUBMED Abstract]
- Argani P, Faria PA, Epstein JI, et al.: Primary renal synovial sarcoma: molecular and morphologic delineation of an entity previously included among embryonal sarcomas of the kidney. Am J Surg Pathol 24 (8): 1087-96, 2000.[PUBMED Abstract]
- Anaplastic Sarcoma of the Kidney
-
General Information About Anaplastic Sarcoma of the Kidney
Anaplastic sarcoma of the kidney is a rare renal tumor that has been identified mainly in patients younger than 15 years.
Patients present with a renal mass, with the most common sites of metastases being the lungs, liver, and bones. (Refer to the Clinical Features of Wilms Tumor and Diagnostic and Staging Evaluation for Wilms Tumor sections of this summary for more information about the clinical features and diagnostic evaluation of childhood kidney tumors.)
Cytogenetic abnormalities such as rearrangement between 10q21 and 18p11.2 have been reported.[ 1 ] These tumors show pathologic features similar to those of pleuropulmonary blastoma of childhood (refer to the PDQ summary on Childhood Pleuropulmonary Blastoma Treatment for more information) and undifferentiated embryonal sarcoma of the liver (refer to the Treatment Options for Undifferentiated Embryonal Sarcoma of the Liver section in the PDQ summary on Childhood Liver Cancer Treatment for more information). Because of the relationship between pleuropulmonary blastoma and renal sarcomas, genetic counseling and testing for a germline DICER1 mutation should be considered. Screening for lung lesions of a solid or cystic nature should also be considered on the basis of age and DICER1 mutation testing.[ 2 ]
Treatment of Anaplastic Sarcoma of the Kidney
There is no standard treatment option for anaplastic sarcoma of the kidney. In the past, these tumors have been identified as anaplastic Wilms tumor and treated accordingly.[ 3 ]
参考文献- Gomi K, Hamanoue S, Tanaka M, et al.: Anaplastic sarcoma of the kidney with chromosomal abnormality: first report on cytogenetic findings. Hum Pathol 41 (10): 1495-9, 2010.[PUBMED Abstract]
- Doros LA, Rossi CT, Yang J, et al.: DICER1 mutations in childhood cystic nephroma and its relationship to DICER1-renal sarcoma. Mod Pathol 27 (9): 1267-80, 2014.[PUBMED Abstract]
- Vujanić GM, Kelsey A, Perlman EJ, et al.: Anaplastic sarcoma of the kidney: a clinicopathologic study of 20 cases of a new entity with polyphenotypic features. Am J Surg Pathol 31 (10): 1459-68, 2007.[PUBMED Abstract]
- Nephroblastomatosis
-
General Information About Nephroblastomatosis (Diffuse Hyperplastic Perilobar Nephroblastomatosis)
Some multifocal nephrogenic rests may become hyperplastic, which may produce a thick rind of blastemal or tubular cells that enlarge the kidney. Radiological studies may be helpful in making the difficult distinction between diffuse hyperplastic perilobar nephroblastomatosis and Wilms tumor. On magnetic resonance imaging, nephrogenic rests appear homogeneous and hypointense with contrast, whereas Wilms tumor has mixed echogenicity and inhomogeneous appearance. Incisional biopsies are difficult to interpret, and it is essential that the biopsy includes the juncture between the lesion and surrounding renal parenchyma.[ 1 ] Differentiation may occur after chemotherapy is administered.
Treatment of Nephroblastomatosis (Diffuse Hyperplastic Perilobar Nephroblastomatosis)
Treatment options for diffuse hyperplastic perilobar nephroblastomatosis include the following:
- Preoperative chemotherapy.
- Renal-sparing surgery. Given the high incidence of bilaterality and subsequent Wilms tumors, renal-sparing surgery may be indicated.[ 1 ]
Evidence (preoperative chemotherapy and surgery):
- In a series of 52 patients with diffuse hyperplastic perilobar nephroblastomatosis, 33 patients were treated with chemotherapy and/or radiation therapy initially, 16 patients underwent unilateral nephrectomy, and 3 patients were observed only.[
1
]
- A total of 24 patients developed Wilms tumor (including the 3 patients who were observed only), at a median of 30 months.
- Eighteen of the 33 patients who received adjuvant chemotherapy alone developed a Wilms tumor.
- Of 16 patients who underwent a nephrectomy and adjuvant therapy, 3 developed Wilms tumor, despite the fact that 14 of 16 patients had bilateral disease.
- Thirty-three percent of the patients who developed Wilms tumor had anaplastic Wilms tumor at some time during their course, probably as a result of selection of chemotherapy-resistant tumors; thus, early detection is critical.
On the basis of this report, it is recommended that patients with diffuse hyperplastic perilobar nephroblastomatosis are monitored by imaging at a maximum interval of 3 months, for a minimum of 7 years; complete resection of growing lesions should be strongly considered because of this high incidence of anaplasia after chemotherapy.[ 1 ]
参考文献- Perlman EJ, Faria P, Soares A, et al.: Hyperplastic perilobar nephroblastomatosis: long-term survival of 52 patients. Pediatr Blood Cancer 46 (2): 203-21, 2006.[PUBMED Abstract]
- Treatment of Recurrent Childhood Kidney Tumors
-
Patients with recurrent rhabdoid tumor of the kidney, clear cell sarcoma of the kidney, neuroepithelial tumor of the kidney, and renal cell carcinoma should be considered for treatment on available phase I and phase II clinical trials.
Regardless of whether a decision is made to pursue disease-directed therapy at the time of progression, palliative care remains a central focus of management. This ensures that quality of life is maximized while attempting to reduce symptoms and stress related to the terminal illness.
Table 9 describes the treatment options for recurrent childhood kidney tumors.
Table 9. Treatment Options for Recurrent Childhood Kidney Tumors Tumor Type Treatment Options Standard-risk relapsed Wilms tumor Surgery, radiation therapy, and chemotherapy High-risk and very high-risk relapsed Wilms tumor Chemotherapy, surgery, and/or radiation therapy Hematopoietic stem cell transplantation Recurrent clear cell sarcoma of the kidney Chemotherapy, surgery, and/or radiation therapy Recurrent congenital mesoblastic nephroma Surgery, chemotherapy, and radiation therapy Prognosis, Prognostic Factors, and Risk Categories for Recurrent Wilms Tumor
Approximately 15% of patients with favorable histology (FH) Wilms tumor and 50% of patients with anaplastic histology Wilms tumor experience recurrence.[ 1 ] The most common site of relapse is lung, followed by abdomen/flank and liver. Recurrence in the brain (0.5%) or bone is rare in children with Wilms tumor.[ 2 ][ 3 ] Historically, the salvage rate for patients with recurrent FH Wilms tumor was 25% to 40%. As a result of modern treatment combinations, the outcome after recurrence has improved up to 60%.[ 4 ][ 5 ]
A number of potential prognostic features influencing postrecurrence outcome have been analyzed, but it is difficult to determine whether these factors are independent of each other. Also, the following prognostic factors appear to be changing as therapy for primary and recurrent Wilms tumor evolves:
The National Wilms Tumor Study (NWTS)-5 trial (NWTS-5 [COG-Q9401/NCT00002611]) showed that time to recurrence and site of recurrence are no longer prognostically significant.[ 4 ][ 7 ] However, in an International Society of Pediatric Oncology (SIOP) study, patients who experienced a pulmonary relapse within 12 months of diagnosis had a poorer prognosis (5-year overall survival [OS] rate, 47%) than did patients who experienced a pulmonary relapse 12 months or more after diagnosis (5-year OS rate, 75%).[ 8 ]
On the basis of these results, the following three risk categories have been identified:
- Standard risk: Patients with FH Wilms tumor who relapse after therapy with only vincristine and/or dactinomycin. These patients are expected to have an event-free survival (EFS) rate of 70% to 80%.[ 5 ] This group represents approximately 30% of recurrences.
- High risk: Patients with FH Wilms tumor who relapse after therapy with three or more agents. These patients account for 45% to 50% of children with Wilms tumor who relapse and have survival rates in the 40% to 50% range.[ 5 ]
- Very high risk: Patients with recurrent anaplastic or blastemal-predominant Wilms tumor. These patients are expected to have survival rates in the 10% range, and they experience 10% to 15% of all Wilms tumor relapses.[ 5 ][ 9 ]
Treatment of Standard-Risk Relapsed Wilms Tumor
In children who had small stage I Wilms tumor and were treated with surgery alone, the EFS rate was 84%. All but one child who relapsed was salvaged with treatment tailored to the site of recurrence.[ 7 ][ 10 ]
Successful retreatment can be accomplished for Wilms tumor patients whose initial therapy consisted of immediate nephrectomy followed by chemotherapy with vincristine and dactinomycin and who relapse.
Treatment options for standard-risk relapsed Wilms tumor include the following:
Surgery, radiation therapy, and chemotherapy
Evidence (surgery, radiation therapy, and chemotherapy):
- Fifty-eight patients were treated on the NWTS-5 relapse protocol with surgical excision when feasible, radiation therapy, and alternating courses of vincristine, doxorubicin, and cyclophosphamide; and etoposide and cyclophosphamide.[
7
]
- The 4-year EFS rate after relapse was 71%, and the OS rate was 82%.
- For patients whose site of relapse was only the lungs, the 4-year EFS rate was 68%, and the OS rate was 81%.
Treatment of High-Risk and Very High-Risk Relapsed Wilms Tumor
Treatment options for high-risk and very high-risk relapsed Wilms tumor include the following:
Chemotherapy, surgery, and/or radiation therapy
Evidence (chemotherapy, surgery, and/or radiation therapy):
- Approximately 50% of unilateral Wilms tumor patients who relapse or progress after initial treatment with vincristine, dactinomycin, and doxorubicin and radiation therapy can be successfully re-treated. Sixty patients with unilateral Wilms tumor were treated on the NWTS-5 relapse protocol with alternating courses of cyclophosphamide/etoposide and carboplatin/etoposide, surgery, and radiation therapy.[
4
][Level of evidence: 2A]
- The 4-year EFS rate for patients with high-risk Wilms tumor was 42%, and the OS rate was 48%.
- High-risk patients who relapsed in the lungs only had a 4-year EFS rate of 49% and an OS rate of 53%.
Patients with stage II, stage III, and stage IV anaplastic tumors at diagnosis have a very poor prognosis upon recurrence.[ 9 ] The combination of ifosfamide, etoposide, and carboplatin demonstrated activity in this group of patients, but significant hematologic toxic effects have been observed.[ 11 ]
HSCT
High-dose chemotherapy followed by autologous HSCT has been utilized for recurrent high-risk patients.[ 12 ][ 13 ]; [ 14 ][Level of evidence: 3ii]
Evidence (HSCT):
- The outcomes of 253 patients with relapsed Wilms tumor who received high-dose chemotherapy followed by autologous HSCT between 1990 and 2013 were reported to and reviewed by the Center for International Blood and Marrow Transplantation Research.[
15
]
- The 5-year estimate for EFS was 36%, and the 5-year estimate for OS was 45%.
- Relapse of primary disease was the cause of death in 81% of the population.
- In a single-institution series of 24 patients with relapsed and refractory Wilms tumor who were treated with high-dose chemotherapy followed by autologous stem cell rescue (HD-ASCR), the following results were reported:[
14
][Level of evidence: 3ii]
- The 3-year disease-free survival (DFS) and OS rates were 46% and 60%, respectively; the 5-year DFS and OS rates were 40% and 54%, respectively. These rates are similar to those reported for conventional salvage therapies.
- No survival advantage was identified on the basis of time to relapse, disease state at time of HD-ASCR, initial stage, or site of relapse.
- No difference was found on the basis of age of diagnosis, sex, histology, or treatment with one versus two cycles of HD-ASCR.
No randomized trials of chemotherapy versus transplant have been reported, and case series suffer from selection bias.
Patients in whom such salvage attempts fail should be offered treatment on available phase I or phase II studies.
Treatment Options Under Clinical Evaluation for Recurrent Wilms Tumor
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
-
APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified using a next-generation sequencing targeted assay of more than 4,000 different mutations across more than 160 genes in refractory and recurrent solid tumors. Children and adolescents aged 1 to 21 years are eligible for the trial.
Tumor tissue from progressive or recurrent disease must be available for molecular characterization. Patients with tumors that have molecular variants addressed by treatment arms included in the trial will be offered treatment on Pediatric MATCH. Additional information can be obtained on the NCI website and ClinicalTrials.gov website.
Treatment of Recurrent Clear Cell Sarcoma of the Kidney
Clear cell sarcoma of the kidney has been characterized by late relapses. However, in trials after 1992, most relapses occurred within 3 years, and the most common sites of recurrence were the brain and the lungs.[ 16 ][ 17 ] In a series of 37 patients with clear cell sarcoma of the kidney who relapsed, the 5-year EFS rate after relapse was 18%, and the OS rate after relapse was 26%.[ 17 ]
The optimal treatment of relapsed clear cell sarcoma of the kidney has not been established. Treatment of patients with recurrent clear cell sarcoma of the kidney depends on initial therapy and site of recurrence.
Treatment options for recurrent clear cell sarcoma of the kidney include the following:
- Chemotherapy, complete surgical resection (if possible), and/or radiation therapy.
Cyclophosphamide and carboplatin should be considered if not used initially. Patients with recurrent clear cell sarcoma of the kidney, in some cases involving the brain, have responded to treatment with ifosfamide, carboplatin, and etoposide (ICE) coupled with local control consisting of surgical resection, radiation therapy, or both.[ 17 ]; [ 18 ][Level of evidence: 2A]
The use of high-dose chemotherapy followed by HSCT is undefined in patients with recurrent clear cell sarcoma of the kidney. A total of 24 patients with relapsed clear cell sarcoma of the kidney received high-dose chemotherapy followed by autologous HSCT. Of those patients, 12 (50%) were alive without disease after a median of 52 months. It should be noted that patients who had already achieved a second complete remission were more likely to receive high-dose chemotherapy.[ 13 ][ 17 ][ 18 ]
Treatment options under clinical evaluation for recurrent clear cell sarcoma of the kidney
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
-
APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified using a next-generation sequencing targeted assay of more than 4,000 different mutations across more than 160 genes in refractory and recurrent solid tumors. Children and adolescents aged 1 to 21 years are eligible for the trial.
Tumor tissue from progressive or recurrent disease must be available for molecular characterization. Patients with tumors that have molecular variants addressed by treatment arms included in the trial will be offered treatment on Pediatric MATCH. Additional information can be obtained on the NCI website and ClinicalTrials.gov website.
Treatment of Recurrent Congenital Mesoblastic Nephroma
Relapses were reported in 4% of patients with congenital mesoblastic nephroma, and all relapses occurred within 12 months after diagnosis. Most relapses occur locally, although metastatic relapses have been reported.[ 19 ] About 70% of patients who relapsed survived with individualized treatment comprising combinations of surgery, chemotherapy, and radiation therapy.[ 19 ]
Targeted therapy should be considered for patients with recurrent or refractory disease containing the ETV6-NTRK3 fusion. Larotrectinib and entrectinib are NTRK inhibitors that are approved for adult and pediatric patients with solid tumors that have an NTRK gene fusion without a known acquired resistance mutation, who are either metastatic or when surgical resection is likely to result in severe morbidity, and who have no satisfactory alternative treatments or whose cancer has progressed after treatment.[ 20 ][ 21 ]
Treatment options under clinical evaluation for recurrent congenital mesoblastic nephroma
Information about NCI-supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following are examples of national and/or institutional clinical trials that are currently being conducted:
-
APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified using a next-generation sequencing targeted assay of more than 4,000 different mutations across more than 160 genes in refractory and recurrent solid tumors. Children and adolescents aged 1 to 21 years are eligible for the trial.
Tumor tissue from progressive or recurrent disease must be available for molecular characterization. Patients with tumors that have molecular variants addressed by treatment arms included in the trial will be offered treatment on Pediatric MATCH. Additional information can be obtained on the NCI website and ClinicalTrials.gov website.
The cellular subtype of congenital mesoblastic nephroma, which commonly harbors the ETV6-NTRK3 fusion, is associated with relapsed disease. Patients should consider enrolling on this trial because one of the treatment arms (APEC1621A [NCT03213704]) uses larotrectinib, which inhibits NTRK fusions.
- LOXO-TRK-15003 (NCT02637687) (Oral TRK Inhibitor LOXO-101 for Treatment of Advanced Pediatric Solid or Primary Central Nervous System [CNS] Tumors): This is a multicenter, open-label, phase I study of pediatric patients with advanced solid or primary CNS tumors. LOXO-101 will be administered orally twice daily, with the dose adjusted by body surface area.
- RXDX-101-03 (NCT02650401) (Study of RXDX-101 in Children With Recurrent or Refractory Solid Tumors and Primary CNS Tumors): This is a four-part, open-label, phase I/Ib, dose-escalation study in pediatric patients with relapsed or refractory solid tumors, primary CNS tumors, neuroblastoma, and non-neuroblastoma, extracranial solid tumors with NTRK1/2/3, ROS1, or ALK gene rearrangements. The study is designed to explore the safety, maximum tolerated dose or recommended phase II dose, pharmacokinetics, and antitumor activity of entrectinib (RXDX-101).
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
参考文献- Green DM, Breslow NE, Beckwith JB, et al.: Effect of duration of treatment on treatment outcome and cost of treatment for Wilms' tumor: a report from the National Wilms' Tumor Study Group. J Clin Oncol 16 (12): 3744-51, 1998.[PUBMED Abstract]
- Venkatramani R, Chi YY, Coppes MJ, et al.: Outcome of patients with intracranial relapse enrolled on national Wilms Tumor Study Group clinical trials. Pediatr Blood Cancer 64 (7): , 2017.[PUBMED Abstract]
- Iaboni DSM, Chi YY, Kim Y, et al.: Outcome of Wilms tumor patients with bone metastasis enrolled on National Wilms Tumor Studies 1-5: A report from the Children's Oncology Group. Pediatr Blood Cancer 66 (1): e27430, 2019.[PUBMED Abstract]
- Malogolowkin M, Cotton CA, Green DM, et al.: Treatment of Wilms tumor relapsing after initial treatment with vincristine, actinomycin D, and doxorubicin. A report from the National Wilms Tumor Study Group. Pediatr Blood Cancer 50 (2): 236-41, 2008.[PUBMED Abstract]
- Reinhard H, Schmidt A, Furtwängler R, et al.: Outcome of relapses of nephroblastoma in patients registered in the SIOP/GPOH trials and studies. Oncol Rep 20 (2): 463-7, 2008.[PUBMED Abstract]
- Grundy P, Breslow N, Green DM, et al.: Prognostic factors for children with recurrent Wilms' tumor: results from the Second and Third National Wilms' Tumor Study. J Clin Oncol 7 (5): 638-47, 1989.[PUBMED Abstract]
- Green DM, Cotton CA, Malogolowkin M, et al.: Treatment of Wilms tumor relapsing after initial treatment with vincristine and actinomycin D: a report from the National Wilms Tumor Study Group. Pediatr Blood Cancer 48 (5): 493-9, 2007.[PUBMED Abstract]
- Warmann SW, Furtwängler R, Blumenstock G, et al.: Tumor biology influences the prognosis of nephroblastoma patients with primary pulmonary metastases: results from SIOP 93-01/GPOH and SIOP 2001/GPOH. Ann Surg 254 (1): 155-62, 2011.[PUBMED Abstract]
- Dome JS, Cotton CA, Perlman EJ, et al.: Treatment of anaplastic histology Wilms' tumor: results from the fifth National Wilms' Tumor Study. J Clin Oncol 24 (15): 2352-8, 2006.[PUBMED Abstract]
- Shamberger RC, Anderson JR, Breslow NE, et al.: Long-term outcomes for infants with very low risk Wilms tumor treated with surgery alone in National Wilms Tumor Study-5. Ann Surg 251 (3): 555-8, 2010.[PUBMED Abstract]
- Abu-Ghosh AM, Krailo MD, Goldman SC, et al.: Ifosfamide, carboplatin and etoposide in children with poor-risk relapsed Wilms' tumor: a Children's Cancer Group report. Ann Oncol 13 (3): 460-9, 2002.[PUBMED Abstract]
- Garaventa A, Hartmann O, Bernard JL, et al.: Autologous bone marrow transplantation for pediatric Wilms' tumor: the experience of the European Bone Marrow Transplantation Solid Tumor Registry. Med Pediatr Oncol 22 (1): 11-4, 1994.[PUBMED Abstract]
- Pein F, Michon J, Valteau-Couanet D, et al.: High-dose melphalan, etoposide, and carboplatin followed by autologous stem-cell rescue in pediatric high-risk recurrent Wilms' tumor: a French Society of Pediatric Oncology study. J Clin Oncol 16 (10): 3295-301, 1998.[PUBMED Abstract]
- Rossoff J, Tse WT, Duerst RE, et al.: High-dose chemotherapy and autologous hematopoietic stem-cell rescue for treatment of relapsed and refractory Wilms tumor: Re-evaluating outcomes. Pediatr Hematol Oncol 35 (5-6): 316-321, 2018 Aug - Sep.[PUBMED Abstract]
- Malogolowkin MH, Hemmer MT, Le-Rademacher J, et al.: Outcomes following autologous hematopoietic stem cell transplant for patients with relapsed Wilms' tumor: a CIBMTR retrospective analysis. Bone Marrow Transplant 52 (11): 1549-1555, 2017.[PUBMED Abstract]
- Seibel NL, Sun J, Anderson JR, et al.: Outcome of clear cell sarcoma of the kidney (CCSK) treated on the National Wilms Tumor Study-5 (NWTS). [Abstract] J Clin Oncol 24 (Suppl 18): A-9000, 502s, 2006.[PUBMED Abstract]
- Gooskens SL, Furtwängler R, Spreafico F, et al.: Treatment and outcome of patients with relapsed clear cell sarcoma of the kidney: a combined SIOP and AIEOP study. Br J Cancer 111 (2): 227-33, 2014.[PUBMED Abstract]
- Radulescu VC, Gerrard M, Moertel C, et al.: Treatment of recurrent clear cell sarcoma of the kidney with brain metastasis. Pediatr Blood Cancer 50 (2): 246-9, 2008.[PUBMED Abstract]
- Gooskens SL, Houwing ME, Vujanic GM, et al.: Congenital mesoblastic nephroma 50 years after its recognition: A narrative review. Pediatr Blood Cancer 64 (7): , 2017.[PUBMED Abstract]
- Drilon A, Laetsch TW, Kummar S, et al.: Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 378 (8): 731-739, 2018.[PUBMED Abstract]
- Entrectinib Shows Pediatric Potential. Cancer Discov 9 (7): OF4, 2019.[PUBMED Abstract]
- Special Considerations for the Treatment of Children With Cancer
-
Cancer in children and adolescents is rare, although the overall incidence of childhood cancer has been slowly increasing since 1975.[ 1 ] Children and adolescents with cancer need to be referred to medical centers that have multidisciplinary teams of cancer specialists with experience treating the cancers that occur during childhood and adolescence. This multidisciplinary team approach incorporates the skills of the following health care professionals and others to ensure that children receive treatment, supportive care, and rehabilitation that will achieve optimal survival and quality of life:
- Primary care physicians.
- Pediatric surgical subspecialists.
- Radiation oncologists.
- Pediatric medical oncologists/hematologists.
- Rehabilitation specialists.
- Pediatric nurse specialists.
- Social workers.
Refer to the PDQ summaries on Supportive and Palliative Care for specific information about supportive care for children and adolescents with cancer.
Guidelines for pediatric cancer centers and their role in the treatment of pediatric patients with cancer have been outlined by the American Academy of Pediatrics.[ 2 ] At these pediatric cancer centers, clinical trials are available for most of the types of cancer that occur in children and adolescents, and the opportunity to participate in these trials is offered to most patients and their families. Clinical trials for children and adolescents with cancer are generally designed to compare potentially better therapy with therapy that is currently accepted as standard. Most of the progress made in identifying curative therapies for childhood cancers has been achieved through clinical trials under the auspices of cooperative groups such as the Children's Oncology Group (COG) and the International Society of Pediatric Oncology (SIOP). Information about ongoing clinical trials is available from the NCI website.
参考文献- Smith MA, Seibel NL, Altekruse SF, et al.: Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28 (15): 2625-34, 2010.[PUBMED Abstract]
- Corrigan JJ, Feig SA; American Academy of Pediatrics: Guidelines for pediatric cancer centers. Pediatrics 113 (6): 1833-5, 2004.[PUBMED Abstract]
- Changes to This Summary (06/08/2020)
-
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Wilms Tumor
Revised Table 2 to include the descriptions of regimen UH1 and regimen UH2 (cited Daw et al. as reference 220).
Revised text about the radiation doses used by the Children's Oncology Group (COG). Also added text to state that the radiation therapy regimen used in the COG AREN0321 trial remains the current standard of treatment.
Added text about the radiation therapy approach used by the International Society of Pediatric Oncology (SIOP).
Added text about the results of the COG study that reported the outcome for patients of all ages with stage I favorable-histology Wilms tumors showing epithelial-predominant histology (cited Parsons et al. as reference 228 and level of evidence 3iiiA).
Revised Table 5 to include the outcome results for patients with stage II diffuse anaplastic Wilms tumors in the COG AREN0321 study.
Revised Table 6 to include the outcome results for patients with stage III diffuse anaplastic Wilms tumors in the COG AREN0321 study.
Revised Table 7 to include the outcome results for patients with stage IV diffuse anaplastic Wilms tumors in the COG AREN0321 study.
Added text about the results of the AREN0321 study that tested the combination of vincristine and irinotecan in an upfront window for patients with diffuse anaplastic Wilms tumor and measurable disease (added level of evidence 3iiiDii).
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
- About This PDQ Summary
-
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of Wilms tumor and other childhood kidney tumors. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
- Louis S. Constine, MD (James P. Wilmot Cancer Center at University of Rochester Medical Center)
- Christopher N. Frantz, MD (Alfred I. duPont Hospital for Children)
- Andrea A. Hayes-Jordan, MD, FACS, FAAP (University of North Carolina - Chapel Hill School of Medicine)
- Nita Louise Seibel, MD (National Cancer Institute)
- Stephen J. Shochat, MD (St. Jude Children's Research Hospital)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Wilms Tumor and Other Childhood Kidney Tumors Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/kidney/hp/wilms-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389282]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.

 画像を拡大する
画像を拡大する