ご利用について
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood thyroid cancer. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
CONTENTS
- Incidence
-
The annual incidence of thyroid cancers is 4.8 to 5.9 cases per 1 million people aged 0 to 19 years, accounting for approximately 1.5% of all cancers in this age group.[ 1 ][ 2 ] Thyroid cancer incidence is higher in children aged 15 to 19 years (17.6 cases per 1 million people), and it accounts for approximately 8% of cancers arising in this older age group.[ 1 ][ 3 ] More thyroid carcinomas occur in females than in males.[ 4 ] The trend toward larger tumors suggests that diagnostic scrutiny is not the only explanation for the observed results.[ 5 ]
Two time-trend studies using the Surveillance, Epidemiology, and End Results (SEER) database have shown a 2% and 3.8% annual increase in the incidence of differentiated thyroid carcinoma in the United States among children, adolescents, and young adults in the 1973 to 2011 and 1984 to 2010 periods, respectively.[ 1 ][ 5 ] A similar trend towards an increase in the incidence of thyroid cancer among children, adolescents, and young adults over the last two decades has been documented in Canada.[ 6 ] A Danish population-based study of thyroid cancer in patients younger than 24 years reported that the age-adjusted incidence rate (per 100,000) increased significantly from 0.36 in 1980 to 0.97 in 2014, with an average annual percent change of 2.9%.[ 7 ] No change in overall survival was observed, but a significant increase was seen in the incidence of thyroid cancer among young adults (aged 18–24 years), mainly attributed to an increase among females and patients with papillary carcinoma.
The incidence of thyroid cancer is higher in whites (5.3 cases per 1 million vs. 1.5 cases per 1 million in blacks) and female adolescents (8.1 cases per 1 million vs. 1.7 cases per 1 million in male adolescents).[ 1 ]
The papillary subtype is the most common, accounting for approximately 60% of the cases, followed by the papillary follicular variant subtype (20%–25%), the follicular subtype (10%), and the medullary subtype (<10%).The incidence of the papillary subtype and its follicular variant peaks between the ages of 15 and 19 years. The incidence of medullary thyroid cancer is the highest in the age group of 0 to 4 years and declines at older ages (refer to Figure 1).[ 2 ]
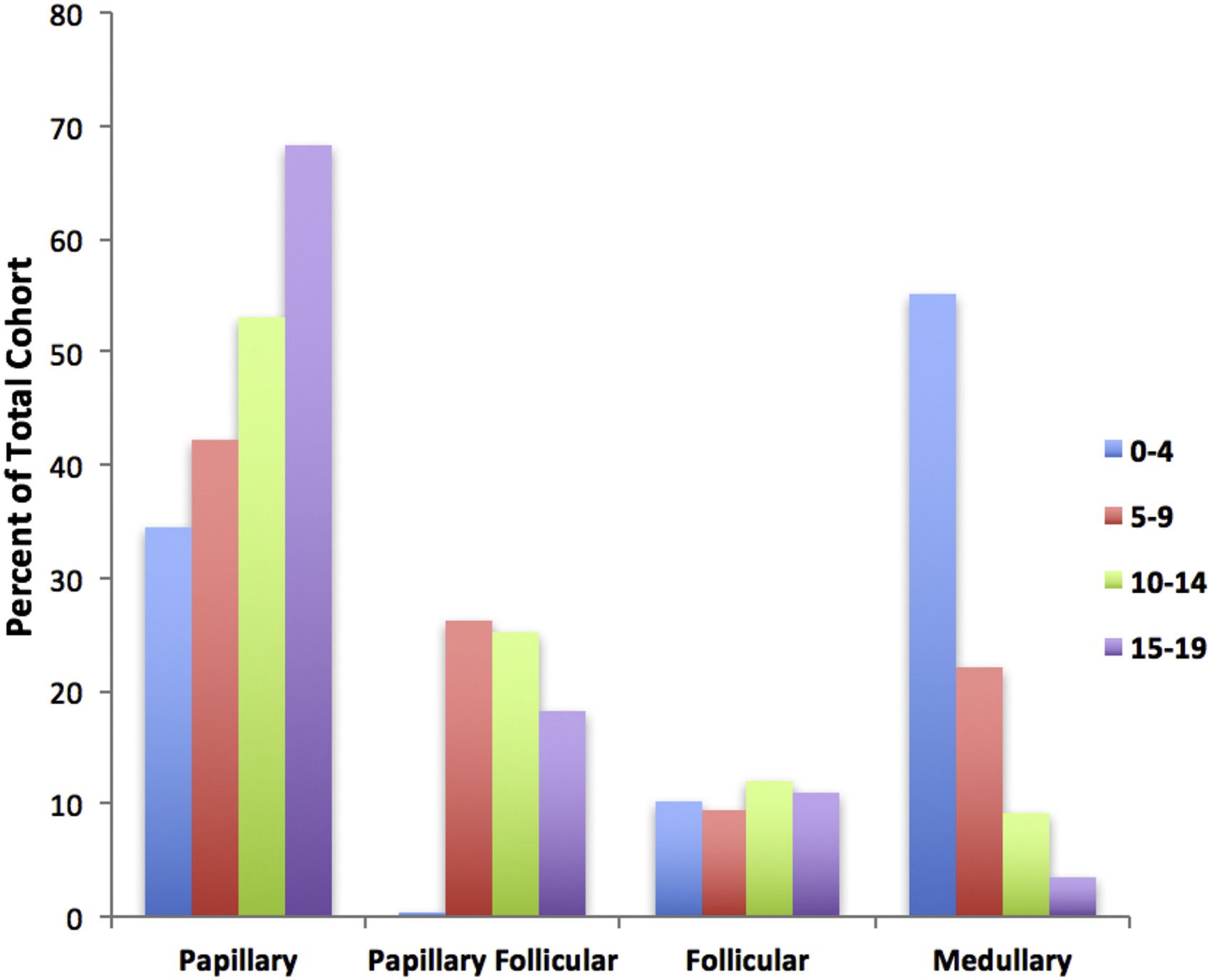
Figure 1. Incidence of pediatric thyroid carcinoma based on most frequent subtype per 100,000 as a percent of total cohort. Reprinted from International Journal of Pediatric Otorhinolaryngology, Volume 89, Sarah Dermody, Andrew Walls, Earl H. Harley Jr., Pediatric thyroid cancer: An update from the SEER database 2007–2012, Pages 121–126, Copyright (2016), with permission from Elsevier. 参考文献- Golpanian S, Perez EA, Tashiro J, et al.: Pediatric papillary thyroid carcinoma: outcomes and survival predictors in 2504 surgical patients. Pediatr Surg Int 32 (3): 201-8, 2016.[PUBMED Abstract]
- Dermody S, Walls A, Harley EH: Pediatric thyroid cancer: An update from the SEER database 2007-2012. Int J Pediatr Otorhinolaryngol 89: 121-6, 2016.[PUBMED Abstract]
- Horner MJ, Ries LA, Krapcho M, et al.: SEER Cancer Statistics Review, 1975-2006. Bethesda, Md: National Cancer Institute, 2009. Also available online. Last accessed January 31, 2020.[PUBMED Abstract]
- Shapiro NL, Bhattacharyya N: Population-based outcomes for pediatric thyroid carcinoma. Laryngoscope 115 (2): 337-40, 2005.[PUBMED Abstract]
- Vergamini LB, Frazier AL, Abrantes FL, et al.: Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr 164 (6): 1481-5, 2014.[PUBMED Abstract]
- Pole JD, Zuk AM, Wasserman JD: Diagnostic and Treatment Patterns Among Children, Adolescents, and Young Adults with Thyroid Cancer in Ontario: 1992-2010. Thyroid 27 (8): 1025-1033, 2017.[PUBMED Abstract]
- Schmidt Jensen J, Grønhøj C, Mirian C, et al.: Incidence and Survival of Thyroid Cancer in Children, Adolescents, and Young Adults in Denmark: A Nationwide Study from 1980 to 2014. Thyroid 28 (9): 1128-1133, 2018.[PUBMED Abstract]
- Risk Factors
-
Risk factors for pediatric thyroid cancer include the following:
参考文献- Cahoon EK, Nadyrov EA, Polyanskaya ON, et al.: Risk of Thyroid Nodules in Residents of Belarus Exposed to Chernobyl Fallout as Children and Adolescents. J Clin Endocrinol Metab 102 (7): 2207-2217, 2017.[PUBMED Abstract]
- Rose J, Wertheim BC, Guerrero MA: Radiation treatment of patients with primary pediatric malignancies: risk of developing thyroid cancer as a secondary malignancy. Am J Surg 204 (6): 881-6; discussion 886-7, 2012.[PUBMED Abstract]
- Lal G, Groff M, Howe JR, et al.: Risk of subsequent primary thyroid cancer after another malignancy: latency trends in a population-based study. Ann Surg Oncol 19 (6): 1887-96, 2012.[PUBMED Abstract]
- Lubin JH, Adams MJ, Shore R, et al.: Thyroid Cancer Following Childhood Low-Dose Radiation Exposure: A Pooled Analysis of Nine Cohorts. J Clin Endocrinol Metab 102 (7): 2575-2583, 2017.[PUBMED Abstract]
- Iglesias ML, Schmidt A, Ghuzlan AA, et al.: Radiation exposure and thyroid cancer: a review. Arch Endocrinol Metab 61 (2): 180-187, 2017 Mar-Apr.[PUBMED Abstract]
- Bauer AJ: Molecular Genetics of Thyroid Cancer in Children and Adolescents. Endocrinol Metab Clin North Am 46 (2): 389-403, 2017.[PUBMED Abstract]
- Acquaviva G, Visani M, Repaci A, et al.: Molecular pathology of thyroid tumours of follicular cells: a review of genetic alterations and their clinicopathological relevance. Histopathology 72 (1): 6-31, 2018.[PUBMED Abstract]
- Francis GL, Waguespack SG, Bauer AJ, et al.: Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 25 (7): 716-59, 2015.[PUBMED Abstract]
- Histology
-
Tumors of the thyroid are classified as adenomas or carcinomas.[ 1 ][ 2 ][ 3 ] Adenomas are benign, well circumscribed and encapsulated nodules that may cause enlargement of all or part of the gland, which extends to both sides of the neck and can be quite large; some tumors may secrete hormones. Transformation to a malignant carcinoma may occur in some cells, which may grow and spread to lymph nodes in the neck or to the lungs. Approximately 20% of thyroid nodules in children are malignant.[ 1 ][ 4 ]
The following histologies account for the general diagnostic category of carcinoma of the thyroid:
-
Differentiated thyroid carcinoma: Papillary and follicular carcinoma are often referred to as differentiated thyroid carcinoma. The pathological classification of differentiated thyroid carcinomas in children is based on standard definitions set by the World Health Organization, and the criteria are the same for children and adults. Long-term outcomes for children and adolescents with differentiated thyroid carcinoma are excellent, with 10-year survival rates exceeding 95%.[
4
][
5
][
6
]
- Papillary thyroid carcinoma: Papillary thyroid carcinoma accounts for 90% or more of all cases of differentiated thyroid carcinoma occurring during childhood and adolescence. Pediatric papillary thyroid carcinoma may present with a variety of histological variants: classic, solid, follicular, and diffuse sclerosing. Papillary thyroid carcinoma is frequently multifocal and bilateral, and metastasizes to regional lymph nodes in most children. Hematogenous metastases to the lungs occur in up to 25% of cases.[ 4 ]
- Follicular thyroid cancer: Follicular thyroid cancer is uncommon. It is typically a unifocal tumor and more prone to initial hematogenous metastases to lungs and bones. Metastases to regional lymph nodes are uncommon. Histologic variants of follicular thyroid cancer include Hürthle cell (oncocytic), clear cell, and insular (poorly differentiated) carcinoma.[ 4 ]
- Medullary thyroid carcinoma: Medullary thyroid carcinoma is a rare form of thyroid carcinoma that originates from the calcitonin-secreting parafollicular C cells and accounts for less than 10% of all cases of thyroid carcinoma in children.[ 6 ] In children, medullary thyroid carcinoma is usually associated with RET germline mutations in the context of multiple endocrine neoplasia type 2 syndrome.[ 7 ]
- Anaplastic carcinoma: Less than 1% of pediatric thyroid carcinomas are anaplastic carcinoma.
参考文献- Dinauer C, Francis GL: Thyroid cancer in children. Endocrinol Metab Clin North Am 36 (3): 779-806, vii, 2007.[PUBMED Abstract]
- Vasko V, Bauer AJ, Tuttle RM, et al.: Papillary and follicular thyroid cancers in children. Endocr Dev 10: 140-72, 2007.[PUBMED Abstract]
- Halac I, Zimmerman D: Thyroid nodules and cancers in children. Endocrinol Metab Clin North Am 34 (3): 725-44, x, 2005.[PUBMED Abstract]
- Francis GL, Waguespack SG, Bauer AJ, et al.: Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 25 (7): 716-59, 2015.[PUBMED Abstract]
- Golpanian S, Perez EA, Tashiro J, et al.: Pediatric papillary thyroid carcinoma: outcomes and survival predictors in 2504 surgical patients. Pediatr Surg Int 32 (3): 201-8, 2016.[PUBMED Abstract]
- Dermody S, Walls A, Harley EH: Pediatric thyroid cancer: An update from the SEER database 2007-2012. Int J Pediatr Otorhinolaryngol 89: 121-6, 2016.[PUBMED Abstract]
- Viola D, Romei C, Elisei R: Medullary thyroid carcinoma in children. Endocr Dev 26: 202-13, 2014.[PUBMED Abstract]
-
Differentiated thyroid carcinoma: Papillary and follicular carcinoma are often referred to as differentiated thyroid carcinoma. The pathological classification of differentiated thyroid carcinomas in children is based on standard definitions set by the World Health Organization, and the criteria are the same for children and adults. Long-term outcomes for children and adolescents with differentiated thyroid carcinoma are excellent, with 10-year survival rates exceeding 95%.[
4
][
5
][
6
]
- Molecular Features
-
Thyroid Carcinoma of Follicular Cells
Thyroid tumorigenesis and progression of thyroid carcinomas of follicular cells (differentiated thyroid carcinoma, poorly-differentiated papillary thyroid carcinoma, and anaplastic thyroid carcinoma) are defined by a multistep process that results in aberrant activation of the MAPK and/or PI3K/PTEN/AKT signaling pathways. Comprehensive genomic studies performed over the last decade have defined the landscape of these tumors, as well as their genotype-phenotype correlations. Mutations in BRAF and RAS genes are the most common driver events, followed by gene fusions involving RET or NTRK:[ 1 ][ 2 ][ 3 ]
-
BRAF: Point mutations of the BRAF gene are the most common alteration found in thyroid carcinoma; the most common mutation is V600E (95% of BRAF-mutated cases). BRAF mutations are found in 40% to 80% of papillary thyroid carcinomas, and in a lower proportion of poorly-differentiated papillary thyroid carcinoma (5%–35%) and anaplastic thyroid carcinoma (10%–50%).[
1
][
3
]
The presence of BRAF V600E has been associated with extrathyroidal tumor extension and an increased risk of recurrence; however, its prognostic significance is controversial. BRAF V600E tumors appear to show a broadly immunosuppressive profile with high expression of anti–programmed death-ligand 1 (PD-L1).[ 1 ][ 3 ]
A retrospective analysis of 80 Brazilian patients younger than 18 years with papillary thyroid carcinoma identified AGK-BRAF fusions and BRAF V600E point mutations.[ 4 ] AGK-BRAF fusions, found in 19% of pediatric patients with papillary thyroid carcinoma, were associated with distant metastasis and younger age. BRAF V600E mutations, found in 15% of patients with pediatric papillary thyroid carcinoma, were correlated with older age and larger tumor size.
- RAS: Oncogenic RAS activation can occur in any of the RAS family of genes (NRAS, HRAS, and KRAS) although the most frequent alterations are NRAS point mutations. RAS mutations are markers of follicular-patterned thyroid lesions; they are present in 30% to 50% of follicular thyroid carcinoma and in 25% to 45% of follicular variants of papillary thyroid carcinoma, while they are seen in less than 10% of papillary thyroid carcinoma. They are also frequently found in poorly differentiated papillary thyroid carcinoma (20%–50%) and anaplastic thyroid carcinoma (10%–50%) and are believed to promote tumor progression. They have a higher prevalence in areas of iodine deficiency.[ 1 ][ 3 ]
- RET-PTC rearrangements: Multiple RET-PTC rearrangements have been identified in approximately 5% to 25% of papillary thyroid carcinomas and in less than 10% of its follicular variant. They are strongly associated with environmental or therapeutic radiation exposure and are also common among young patients, many of whom present with nodal metastases and aggressive clinicopathological features. [ 1 ][ 3 ]
- NTRK rearrangements: Rearrangements of NTRK1 and NTRK3 have been described in approximately 5% of papillary thyroid carcinomas; however, ETV6-NTRK3 has been reported in 15% of radiation-induced papillary thyroid carcinomas. In young patients and children, NTRK-rearranged papillary thyroid carcinomas may present with lymph node metastases and aggressive clinicopathological features, similar to the presentation of RET-rearranged tumors.[ 1 ][ 3 ]
- DICER1 mutations: Conventional alterations were found in 12 of 30 (40%) papillary thyroid carcinomas (five cases with BRAF V600E, three cases with RET/PTC1, and four cases with RET/PTC3). Pathogenic mutations of DICER1 have been identified in approximately 10% of papillary thyroid carcinomas.[ 5 ]
Other alterations include the following:[ 1 ][ 3 ]
- ALK rearrangements have been described in less than 10% of papillary thyroid carcinomas and are commonly associated with dedifferentiation.
- Activating mutations of AKT1 have been described in 19% of recurrent or metastatic poorly differentiated papillary thyroid carcinoma.
- PPARG rearrangements are present in 20% to 50% of follicular thyroid carcinoma and in a lower proportion of follicular variants of papillary thyroid carcinoma.
- TERT-activating mutations are commonly seen in poorly differentiated papillary thyroid carcinoma (20%–50%) and anaplastic thyroid carcinoma (30%–75%), and have also been reported in 10% to 35% of follicular thyroid carcinomas and 5% to 15% of papillary thyroid carcinomas. TERT mutations are believed to promote tumor progression to poorly differentiated papillary thyroid carcinoma and anaplastic thyroid carcinoma and represent a negative prognostic marker.
- TP53 is mutated in 40% to 80% of anaplastic thyroid carcinomas and 10% to 35% of poorly differentiated papillary thyroid carcinoma; it is considered to be a final step of tumor progression and a marker for poor prognosis.
The spectrum of somatic genetic alterations seems to be different between pediatric and adult patients when analyzing tumors with similar histologies, as follows:[ 2 ]
- Gene fusions involving RET or, less frequently, NTRK account for approximately 50% of the molecular alterations in pediatric differentiated thyroid carcinoma, compared with approximately 15% in adults.
- Point mutations involving BRAF or RAS, which are the defining alterations in approximately 70% of thyroid carcinomas diagnosed in adults, are noted in 30% to 40% of pediatric tumors; BRAF mutations have been described in approximately 30% of cases, while RAS mutations are much less frequently found in pediatrics (5%–10%).
Medullary Thyroid Carcinoma
Medullary thyroid carcinoma is a neuroendocrine malignancy derived from the neural crest-originated parafollicular C cells of the thyroid gland. In children, medullary thyroid carcinoma is a monogenic disorder caused by a dominantly inherited or de novo gain-of-function mutation in the RET oncogene associated with multiple endocrine neoplasia type 2 (MEN2), either MEN2A or MEN2B, depending on the specific mutation. The highest medullary thyroid carcinoma risk is conferred by the RET M918T mutation, which is associated with MEN2B; the RET mutations associated with MEN2A confer a lower medullary thyroid carcinoma risk.[ 2 ]
参考文献- Acquaviva G, Visani M, Repaci A, et al.: Molecular pathology of thyroid tumours of follicular cells: a review of genetic alterations and their clinicopathological relevance. Histopathology 72 (1): 6-31, 2018.[PUBMED Abstract]
- Bauer AJ: Molecular Genetics of Thyroid Cancer in Children and Adolescents. Endocrinol Metab Clin North Am 46 (2): 389-403, 2017.[PUBMED Abstract]
- Cancer Genome Atlas Research Network: Integrated genomic characterization of papillary thyroid carcinoma. Cell 159 (3): 676-90, 2014.[PUBMED Abstract]
- Sisdelli L, Cordioli MICV, Vaisman F, et al.: AGK-BRAF is associated with distant metastasis and younger age in pediatric papillary thyroid carcinoma. Pediatr Blood Cancer 66 (7): e27707, 2019.[PUBMED Abstract]
- Wasserman JD, Sabbaghian N, Fahiminiya S, et al.: DICER1 Mutations Are Frequent in Adolescent-Onset Papillary Thyroid Carcinoma. J Clin Endocrinol Metab 103 (5): 2009-2015, 2018.[PUBMED Abstract]
-
BRAF: Point mutations of the BRAF gene are the most common alteration found in thyroid carcinoma; the most common mutation is V600E (95% of BRAF-mutated cases). BRAF mutations are found in 40% to 80% of papillary thyroid carcinomas, and in a lower proportion of poorly-differentiated papillary thyroid carcinoma (5%–35%) and anaplastic thyroid carcinoma (10%–50%).[
1
][
3
]
- Clinical Presentation and Prognostic Factors
-
Differentiated Thyroid Carcinoma
Patients with thyroid cancer usually present with a thyroid mass with or without painless cervical adenopathy.[ 1 ] On the basis of medical and family history and clinical constellation, the thyroid cancer may be part of a tumor predisposition syndrome such as multiple endocrine neoplasia (MEN), APC-associated polyposis, PTEN hamartoma tumor syndrome, Carney complex, Werner syndrome, and DICER1 syndrome.[ 2 ][ 3 ]
Younger age is associated with a more aggressive clinical presentation in differentiated thyroid carcinoma. The following observations have been reported:
- In a cross-sectional study involving 20% of community hospitals in the United States, the clinical presentation of 644 pediatric cases was compared with that of more than 43,000 adult cases. Compared with adults, children had a higher proportion of nodal involvement (31.5% in children vs. 14.7% in adults) and lung metastases (5.7% in children vs. 2.2% in adults).[ 1 ]
- Higher recurrence rates have been associated with younger age at presentation.[ 4 ]
- Larger tumor size (>1 cm), extrathyroidal extension, and multifocal disease are associated with increased risk of nodal metastases.[ 5 ]
- When compared with pubertal adolescents, prepubertal children have a more aggressive presentation with a greater degree of extrathyroid extension, lymph node involvement, and lung metastases. However, outcome is similar in the prepubertal and adolescent groups.[ 6 ]
In well-differentiated thyroid cancer, male sex, large tumor size, and distant metastases have been found to have prognostic significance for early mortality; however, even patients in the highest risk group who had distant metastases had a 90% survival rate.[ 7 ] A French registry analysis found similar outcomes in children and young adults who developed papillary thyroid carcinoma after previous radiation therapy compared with children and young adults who developed spontaneous papillary thyroid carcinoma; patients with previous thyroid irradiation for benign disease, however, presented with more invasive tumors and lymph node involvement.[ 8 ]
A review of the National Cancer Database found that patients aged 21 years and younger from lower-income families and those lacking insurance experienced a longer period from diagnosis to treatment of their well-differentiated thyroid cancer and presented with higher-stage disease.[ 9 ]
Medullary Thyroid Carcinoma
Children with medullary thyroid carcinoma present with a more aggressive clinical course; 50% of the cases have hematogenous metastases at diagnosis.[ 10 ] A natural history study of children and young adults with medullary thyroid cancer is being conducted by the National Cancer Institute (NCT01660984). A review of 430 patients aged 0 to 21 years with medullary thyroid cancer reported that older age (16–21 years) at diagnosis, tumor diameter greater than 2 cm, positive margins after total thyroidectomy, and lymph node metastases were associated with a worse prognosis.[ 11 ]
From 1997 to 2019, the German Society for Pediatric Oncology and Hematology (GPOH)–Malignant Endocrine Tumors (MET) registry identified a total of 57 patients with medullary thyroid carcinoma and 17 patients with C-cell hyperplasia.[ 12 ][Level of evidence: 3iA] In patients with medullary thyroid carcinoma, the median follow-up was 5 years (range, 0–19 years), and the median age at diagnosis was 10 years (range, 0–17 years). The overall survival rate was 87%, and the event-free survival (EFS) rate was 52%. In total, 96.4% of patients were affected by MEN type 2 (MEN2) syndromes; 37 of 42 patients had MEN2A, and 3 of 28 patients had MEN2B (RET M918T mutation). The 10-year EFS rate was 78% for patients with MEN2A and 38% for patients with MEN2B (P < .001). In multivariate analyses, positive lymph node status and postoperatively elevated calcitonin levels were significant adverse prognostic factors for EFS.
In children with hereditary MEN2B, medullary thyroid carcinoma may be detectable within the first year of life and nodal metastases may occur before age 5 years. The recognition of mucosal neuromas, a history of alacrima, constipation (secondary to intestinal ganglioneuromatosis), and marfanoid facial features and body habitus is critical to early recognition and diagnosis because the RET M918T mutation associated with MEN2B is often de novo. Approximately 50% of patients with MEN2B develop a pheochromocytoma, with a varying degree of risk of developing pheochromocytoma and hyperparathyroidism in MEN2A based on the specific RET mutation.[ 13 ][ 14 ] (Refer to the PDQ summary on Childhood Multiple Endocrine Neoplasia [MEN] Syndromes Treatment for more information.)
For children with de novo RET mutations and no familial history, nonendocrine manifestations, such as intestinal ganglioneuromatosis or skeletal or ocular stigmata, may facilitate early diagnosis and result in better outcomes.[ 14 ]
参考文献- Al-Qurayshi Z, Hauch A, Srivastav S, et al.: A National Perspective of the Risk, Presentation, and Outcomes of Pediatric Thyroid Cancer. JAMA Otolaryngol Head Neck Surg 142 (5): 472-8, 2016.[PUBMED Abstract]
- Acquaviva G, Visani M, Repaci A, et al.: Molecular pathology of thyroid tumours of follicular cells: a review of genetic alterations and their clinicopathological relevance. Histopathology 72 (1): 6-31, 2018.[PUBMED Abstract]
- Francis GL, Waguespack SG, Bauer AJ, et al.: Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 25 (7): 716-59, 2015.[PUBMED Abstract]
- Ye B, Shi J, Shen C, et al.: Comparison of differentiated thyroid carcinoma recurrence and its clinical features in children of different ages. Oncotarget 8 (29): 48051-48059, 2017.[PUBMED Abstract]
- Kim J, Sun Z, Adam MA, et al.: Predictors of nodal metastasis in pediatric differentiated thyroid cancer. J Pediatr Surg 52 (1): 120-123, 2017.[PUBMED Abstract]
- Lazar L, Lebenthal Y, Steinmetz A, et al.: Differentiated thyroid carcinoma in pediatric patients: comparison of presentation and course between pre-pubertal children and adolescents. J Pediatr 154 (5): 708-14, 2009.[PUBMED Abstract]
- Shayota BJ, Pawar SC, Chamberlain RS: MeSS: A novel prognostic scale specific for pediatric well-differentiated thyroid cancer: a population-based, SEER outcomes study. Surgery 154 (3): 429-35, 2013.[PUBMED Abstract]
- Sassolas G, Hafdi-Nejjari Z, Casagranda L, et al.: Thyroid cancers in children, adolescents, and young adults with and without a history of childhood exposure to therapeutic radiation for other cancers. Thyroid 23 (7): 805-10, 2013.[PUBMED Abstract]
- Garner EF, Maizlin II, Dellinger MB, et al.: Effects of socioeconomic status on children with well-differentiated thyroid cancer. Surgery 162 (3): 662-669, 2017.[PUBMED Abstract]
- Waguespack SG, Rich TA, Perrier ND, et al.: Management of medullary thyroid carcinoma and MEN2 syndromes in childhood. Nat Rev Endocrinol 7 (10): 596-607, 2011.[PUBMED Abstract]
- Raval MV, Sturgeon C, Bentrem DJ, et al.: Influence of lymph node metastases on survival in pediatric medullary thyroid cancer. J Pediatr Surg 45 (10): 1947-54, 2010.[PUBMED Abstract]
- Kuhlen M, Frühwald MC, Dunstheimer DPA, et al.: Revisiting the genotype-phenotype correlation in children with medullary thyroid carcinoma: A report from the GPOH-MET registry. Pediatr Blood Cancer 67 (4): e28171, 2020.[PUBMED Abstract]
- Bauer AJ: Molecular Genetics of Thyroid Cancer in Children and Adolescents. Endocrinol Metab Clin North Am 46 (2): 389-403, 2017.[PUBMED Abstract]
- Wells SA, Asa SL, Dralle H, et al.: Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25 (6): 567-610, 2015.[PUBMED Abstract]
- Diagnostic Evaluation
-
Initial evaluation of a child or adolescent with a thyroid nodule includes the following:
- Ultrasound of the thyroid.
- Serum thyroid-stimulating hormone (TSH) level.
- Serum thyroglobulin level.
Tests of thyroid function are usually normal, but thyroglobulin can be elevated.
Fine-needle aspiration as an initial diagnostic approach is sensitive and useful. However, in doubtful cases, open biopsy or resection should be considered.[ 1 ]
参考文献- Francis GL, Waguespack SG, Bauer AJ, et al.: Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 25 (7): 716-59, 2015.[PUBMED Abstract]
- Treatment of Papillary and Follicular Thyroid Carcinoma
-
Treatment options for papillary and follicular (differentiated) thyroid carcinoma include the following:
- Surgery.
- Radioactive iodine ablation.
- Targeted therapy.
In 2015, the American Thyroid Association (ATA) Task Force on Pediatric Thyroid Cancer published guidelines for the management of thyroid nodules and differentiated thyroid cancer in children and adolescents. These guidelines (summarized below) are based on scientific evidence and expert panel opinion, with a careful assessment of the level of evidence.[ 1 ]
- Preoperative evaluation.[
1
]
- A comprehensive ultrasound of all regions of the neck using a high-resolution probe and Doppler technique should be obtained by an experienced ultrasonographer. A complete ultrasound examination should be performed before surgery.
- The addition of cross-sectional imaging (contrast-enhanced computed tomography [CT] or magnetic resonance imaging) should be considered when there is concern about invasion of the aerodigestive tract. Importantly, if iodinated contrast agents are used, further evaluation and treatment with radioactive iodine may need to be delayed for 2 to 3 months until total body iodine burden decreases.
- Chest imaging (x-ray or CT) may be considered for patients with substantial cervical lymph node disease.
- Thyroid nuclear scintigraphy should be pursued only if the patient presents with a suppressed thyroid-stimulating hormone (TSH).
- The routine use of bone scan or fluorine F 18-fludeoxyglucose positron emission tomography (PET) is not recommended.
- Surgery.[
1
]
Pediatric thyroid surgery is ideally completed by a surgeon who has experience performing endocrine procedures in children and in a hospital with the full spectrum of pediatric specialty care.
- Thyroidectomy:
For patients with papillary or follicular carcinoma, total thyroidectomy is the recommended treatment of choice. The ATA expert panel recommendation is based on data showing an increased incidence of bilateral (30%) and multifocal (65%) disease.
In patients with a small unilateral tumor confined to the gland, a near-total thyroidectomy—whereby a small amount of thyroid tissue (<1%–2%) is left in place at the entry point of the recurrent laryngeal nerve or superior parathyroid glands—might be considered to decrease permanent damage to those structures.[ 2 ]
Total thyroidectomy also optimizes the use of radioactive iodine for imaging and treatment.
- Central neck dissection:
- A therapeutic central neck lymph node dissection should be done in the presence of clinical evidence of central or lateral neck metastases.[ 3 ]
- For patients without clinical evidence of gross extrathyroidal invasion or locoregional metastasis, a prophylactic central neck dissection may be considered on the basis of tumor focality and size of the primary tumor. However, because of the increased morbidity associated with central lymph node dissection, it is important to carefully individualize each case on the basis of the risks and benefits of the extent of dissection.[ 4 ]
- Lateral neck dissection:
- Cytological confirmation of metastatic disease to lymph nodes in the lateral neck is recommended before surgery.
- Routine prophylactic lateral neck dissection is not recommended.
- Thyroidectomy:
- Classification and risk assignment.[
1
]
Despite the limited data in pediatrics, the ATA Task Force recommends the use of the tumor-node-metastasis (TNM) classification system to categorize patients into one of three risk groups. (Refer to the Stage Information for Thyroid Cancer section in the PDQ summary on Thyroid Cancer Treatment [Adult] for more information about the TNM system.) This categorization strategy is meant to define the risk of persistent cervical disease and help determine which patients should undergo postoperative staging for the presence of distant metastasis.
- ATA Pediatric Low Risk: Disease confined to the thyroid with N0 or NX disease or patients with incidental N1a (microscopic metastasis to a small number of central neck nodes). These patients are at lowest risk of distant disease but may still be at risk of residual cervical disease, especially if the initial surgery did not include central neck dissection.
- ATA Pediatric Intermediate Risk: Extensive N1a or minimal N1b disease. These patients are at low risk of distant metastasis but are at an increased risk of incomplete lymph node resection and persistent cervical disease.
- ATA Pediatric High Risk: Regionally extensive disease (N1b) or locally invasive disease (T4), with or without distant metastasis. Patients in this group are at the highest risk of incomplete resection, persistent disease, and distant metastasis.
- Postoperative staging and long-term surveillance.[
1
]
Initial staging should be performed within 12 weeks after surgery; the purpose is to assess for evidence of persistent locoregional disease and to identify patients who are likely to benefit from additional therapy with iodine I 131 (131I). The ATA Pediatric Risk Level (as defined above) helps determine the extent of postoperative testing.
- ATA Pediatric Low Risk:
- Initial postoperative staging includes a TSH-suppressed thyroglobulin. A diagnostic iodine I 123 (123I) scan is not required.
- TSH suppression should be targeted to serum levels of 0.5 to 1.0 mIU/L.
- In patients with no evidence of disease, surveillance should include ultrasound at 6 months postoperatively and then annually for 5 years; and thyroglobulin levels (on hormone replacement therapy) every 3 to 6 months for 2 years and then annually.
- ATA Pediatric Intermediate Risk:
- Initial postoperative staging includes a TSH-stimulated thyroglobulin and diagnostic 123I whole-body scan for further stratification and determination with 131I.
- TSH suppression should be targeted to serum levels of 0.1 to 0.5 mIU/L.
- In patients with no evidence of disease, surveillance should include ultrasound at 6 months postoperatively and then every 6 to 12 months for 5 years (and then less frequently); and thyroglobulin levels (on hormone replacement therapy) every 3 to 6 months for 3 years and then annually.
- TSH-stimulated thyroglobulin and diagnostic 123I scan should be considered in 1 to 2 years for patients treated with 131I.
- ATA Pediatric High Risk:
- Initial postoperative staging includes a TSH-stimulated thyroglobulin and diagnostic 123I whole-body scan for further stratification and determination with 131I.
- TSH suppression should be targeted to serum levels of less than 0.1 mIU/L.
- In patients with no evidence of disease, surveillance should include ultrasound at 6 months postoperatively and then every 6 to 12 months for 5 years (and then less frequently); and thyroglobulin levels (on hormone replacement therapy) every 3 to 6 months for 3 years and then annually.
- TSH-stimulated thyroglobulin and, possibly, a diagnostic 123I scan in 1 to 2 years in patients treated with 131I.
For patients with antithyroglobulin antibodies, consideration can be given to deferred postoperative staging to allow time for antibody clearance, except in patients with T4 or M1 disease.
- ATA Pediatric Low Risk:
- Radioactive iodine ablation.[
1
]
The goal of 131I therapy is to decrease the risks of recurrence and to decrease mortality by eliminating iodine-avid disease.
- The ATA Task Force recommends the use of 131I for the treatment of iodine-avid persistent locoregional or nodal disease that cannot be resected and known or presumed iodine-avid distant metastases. For patients with persistent disease after administration of 131I, the decision to pursue additional 131I therapy should be individualized on the basis of clinical data and previous response.
- To facilitate 131I uptake by residual iodine-avid disease, the TSH level should be above 30 mIU/L. This level can be achieved by withdrawing levothyroxine for at least 14 days. In patients who cannot mount an adequate TSH response or cannot tolerate profound hypothyroidism, recombinant human TSH may be used.
- Therapeutic 131I administration is commonly based on either empiric dosing or whole-body dosimetry. Based on the lack of data comparing empiric treatment and treatment informed by dosimetry, the ATA Task Force was unable to recommend one specific approach. However, because of the differences in body size and iodine clearance in children compared with adults, it is recommended that all activities of 131I should be calculated by experts with experience in dosing children.
- A posttreatment whole-body scan is recommended for all children 4 to 7 days after 131I therapy. The addition of single-photon emission CT with integrated conventional CT (SPECT/CT) may help to distinguish the anatomic location of focal uptake.
While rare, late effects of 131I treatment include salivary gland dysfunction, bone marrow suppression, pulmonary fibrosis, and second malignancies.[ 5 ]
Larotrectinib (a targeted therapy) has been used to treat patients with TRK fusion–positive thyroid carcinoma. Five of five patients with TRK fusion–positive thyroid carcinomas who were treated with larotrectinib therapy achieved partial or complete responses.[ 6 ]
参考文献- Francis GL, Waguespack SG, Bauer AJ, et al.: Management Guidelines for Children with Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 25 (7): 716-59, 2015.[PUBMED Abstract]
- Spinelli C, Strambi S, Rossi L, et al.: Surgical management of papillary thyroid carcinoma in childhood and adolescence: an Italian multicenter study on 250 patients. J Endocrinol Invest 39 (9): 1055-9, 2016.[PUBMED Abstract]
- Kim J, Sun Z, Adam MA, et al.: Predictors of nodal metastasis in pediatric differentiated thyroid cancer. J Pediatr Surg 52 (1): 120-123, 2017.[PUBMED Abstract]
- Machens A, Elwerr M, Thanh PN, et al.: Impact of central node dissection on postoperative morbidity in pediatric patients with suspected or proven thyroid cancer. Surgery 160 (2): 484-92, 2016.[PUBMED Abstract]
- Albano D, Bertagna F, Panarotto MB, et al.: Early and late adverse effects of radioiodine for pediatric differentiated thyroid cancer. Pediatr Blood Cancer 64 (11): , 2017.[PUBMED Abstract]
- Drilon A, Laetsch TW, Kummar S, et al.: Efficacy of Larotrectinib in TRK Fusion-Positive Cancers in Adults and Children. N Engl J Med 378 (8): 731-739, 2018.[PUBMED Abstract]
- Treatment of Recurrent Papillary and Follicular Thyroid Carcinoma
-
Despite the more advanced disease at presentation compared with adults, children with differentiated thyroid cancer generally have an excellent survival with relatively few side effects.[ 1 ][ 2 ][ 3 ]
Treatment options for recurrent papillary and follicular thyroid carcinoma include the following:
- Radioactive iodine ablation with iodine I 131 (131I).
Radioactive iodine ablation with 131I is usually effective after recurrence.[ 4 ] For patients with 131I-refractory disease, molecularly targeted therapies using kinase inhibitors may provide alternative therapies.
Tyrosine kinase inhibitors (TKIs) with documented efficacy for the treatment of adults include the following:
-
Sorafenib. Sorafenib is a vascular endothelial growth factor receptor (VEGFR), platelet-derived growth factor receptor (PDGFR), and RAS kinase inhibitor. In a randomized phase III trial, sorafenib improved progression-free survival (PFS) when compared with placebo (10.8 months vs. 5.8 months) in adult patients with radioactive iodine–refractory locally advanced or metastatic differentiated thyroid cancer.[
5
] Sorafenib was approved by the U.S. Food and Drug Administration (FDA) in November 2013 for the treatment of adults with late-stage metastatic differentiated thyroid carcinoma.
Pediatric-specific data are very limited; however, in one case report, sorafenib produced a radiographic response in a patient aged 8 years with metastatic papillary thyroid carcinoma.[ 6 ]
-
Lenvatinib. Lenvatinib is an oral VEGFR, fibroblast growth factor receptor, PDGFR, RET, and KIT inhibitor. In a phase III randomized study of adults with 131I-refractory differentiated thyroid cancer, lenvatinib was associated with a significant improvement in PFS and response rate when compared with a placebo.[
7
] Lenvatinib was approved by the FDA in February 2015 for the treatment of adults with progressive radioactive iodine–refractory differentiated thyroid carcinoma.
Three children with papillary thyroid carcinoma who were refractory to radioactive iodine had a clinical response to lenvatinib.[ 8 ]
- BRAF inhibitors. In an open-label, nonrandomized phase II study of vemurafenib in adult patients with 131I-refractory metastatic or unresectable BRAF-V600E positive papillary thyroid carcinoma who had not been previously treated with a TKI, a response rate of 38.5% was documented.[ 9 ] For patients with metastatic or advanced BRAF V600E–mutated anaplastic thyroid carcinoma, the combination of dabrafenib with the MEK inhibitor trametinib has shown a response rate of 69%.[ 10 ]
(Refer to the PDQ summary on Thyroid Cancer Treatment [Adult] for more information.)
Treatment Options Under Clinical Evaluation for Recurrent Papillary and Follicular Thyroid Carcinoma
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
-
APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified using a next-generation sequencing targeted assay of more than 4,000 different mutations across more than 160 genes in refractory and recurrent solid tumors. Children and adolescents aged 1 to 21 years are eligible for the trial.
Tumor tissue from progressive or recurrent disease must be available for molecular characterization. Patients with tumors that have molecular variants addressed by treatment arms included in the trial will be offered treatment on Pediatric MATCH. Additional information can be obtained on the NCI website and ClinicalTrials.gov website.
参考文献- Golpanian S, Perez EA, Tashiro J, et al.: Pediatric papillary thyroid carcinoma: outcomes and survival predictors in 2504 surgical patients. Pediatr Surg Int 32 (3): 201-8, 2016.[PUBMED Abstract]
- Vergamini LB, Frazier AL, Abrantes FL, et al.: Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr 164 (6): 1481-5, 2014.[PUBMED Abstract]
- Dermody S, Walls A, Harley EH: Pediatric thyroid cancer: An update from the SEER database 2007-2012. Int J Pediatr Otorhinolaryngol 89: 121-6, 2016.[PUBMED Abstract]
- Powers PA, Dinauer CA, Tuttle RM, et al.: Treatment of recurrent papillary thyroid carcinoma in children and adolescents. J Pediatr Endocrinol Metab 16 (7): 1033-40, 2003.[PUBMED Abstract]
- Brose MS, Nutting CM, Jarzab B, et al.: Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384 (9940): 319-28, 2014.[PUBMED Abstract]
- Iyer P, Mayer JL, Ewig JM: Response to sorafenib in a pediatric patient with papillary thyroid carcinoma with diffuse nodular pulmonary disease requiring mechanical ventilation. Thyroid 24 (1): 169-74, 2014.[PUBMED Abstract]
- Schlumberger M, Tahara M, Wirth LJ, et al.: Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372 (7): 621-30, 2015.[PUBMED Abstract]
- Mahajan P, Dawrant J, Kheradpour A, et al.: Response to Lenvatinib in Children with Papillary Thyroid Carcinoma. Thyroid 28 (11): 1450-1454, 2018.[PUBMED Abstract]
- Brose MS, Cabanillas ME, Cohen EE, et al.: Vemurafenib in patients with BRAF(V600E)-positive metastatic or unresectable papillary thyroid cancer refractory to radioactive iodine: a non-randomised, multicentre, open-label, phase 2 trial. Lancet Oncol 17 (9): 1272-82, 2016.[PUBMED Abstract]
- Subbiah V, Kreitman RJ, Wainberg ZA, et al.: Dabrafenib and Trametinib Treatment in Patients With Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J Clin Oncol 36 (1): 7-13, 2018.[PUBMED Abstract]
- Treatment of Medullary Thyroid Carcinoma
-
Medullary thyroid carcinomas are commonly associated with the multiple endocrine neoplasia type 2 (MEN2) syndrome (refer to the PDQ summary on Childhood Multiple Endocrine Neoplasia [MEN] Syndromes Treatment for more information).
Treatment options for medullary thyroid carcinoma include the following:
-
Surgery: Treatment for children with medullary thyroid carcinoma is mainly surgical. Investigators have concluded that prophylactic central node dissection should not be performed on patients with hereditary medullary thyroid cancer if their basal calcitonin serum levels are lower than 40 pg/mL.[
1
]
Most cases of medullary thyroid carcinoma in children occur in the context of the MEN2A and MEN2B syndromes. In those familial cases, early genetic testing and counseling is indicated, and prophylactic surgery is recommended for children with the RET germline mutation. Strong genotype-phenotype correlations have facilitated the development of guidelines for intervention, including screening and age at which prophylactic thyroidectomy should occur.[ 2 ]
A retrospective analysis identified 167 children with RET mutations who underwent prophylactic thyroidectomy; this group included 109 patients without a concomitant central node dissection and 58 patients with a concomitant central node dissection. Postoperative hypoparathyroidism was more frequent in older children (32% in the oldest age group vs. 3% in the youngest age group; P = .002), regardless of whether central node dissection was carried out. Three children developed recurrent laryngeal nerve palsy, all of whom had undergone central node dissection (P = .040). All complications resolved within 6 months. Postoperative normalization of calcitonin serum levels was achieved in 114 of 115 children (99.1%) with raised preoperative values. Children were classified into risk groups by their specific type of RET mutation (refer to Table 1).[ 3 ]
- In the highest-risk category, medullary thyroid carcinoma was found in five of six children (83%) aged 3 years or younger.
- In the high-risk category, medullary thyroid carcinoma was present in 6 of 20 children (30%) aged 3 years or younger, 16 of 36 children (44%) aged 4 to 6 years, and 11 of 16 children (69%) aged 7 to 12 years (P = .081).
- In the moderate-risk category, medullary thyroid carcinoma was seen in one of nine children (11%) aged 3 years or younger, 1 of 26 children (4%) aged 4 to 6 years, 3 of 26 children (12%) aged 7 to 12 years, and 7 of 16 children (44%) aged 13 to 18 years (P = .006).
The American Thyroid Association has proposed the following guidelines for prophylactic thyroidectomy in children with hereditary medullary thyroid carcinoma (refer to Table 1).[ 2 ]
Table 1. Risk Levels and Management Based on Common RET Mutations Detected on Genetic Screeninga Medullary Thyroid Carcinoma Risk Level Highest (MEN2B) High (MEN2A) Moderate (MEN2A) MEN2A = multiple endocrine neoplasia type 2A; MEN2B = multiple endocrine neoplasia type 2B. aAdapted from Wells et al.[ 2 ] RET Mutation M918T A883F, C634F/G/R/S/W/Y G533C, C609F/G/R/S/Y, C611F/G/S/Y/W, C618F/R/S, C620F/R/S, C630R/Y, D631Y, K666E, E768D, L790F, V804L, V804M, S891A, R912P Age for Prophylactic Thyroidectomy Total thyroidectomy in the first year of life, ideally in the first months of life. Total thyroidectomy at or before age 5 y based on serum calcitonin levels. Total thyroidectomy to be performed when the serum calcitonin level is above the normal range or at convenience if the parents do not wish to embark on a lengthy period of surveillance. -
Tyrosine kinase inhibitor (TKI) therapy: A number of TKIs have been evaluated and approved for patients with advanced thyroid carcinoma.
-
Vandetanib. Vandetanib (an inhibitor of RET kinase, vascular endothelial growth factor receptor [VEGFR], and epidermal growth factor receptor signaling) is approved by the U.S. Food and Drug Administration (FDA) for the treatment of symptomatic or progressive medullary thyroid cancer in adult patients with unresectable, locally advanced, or metastatic disease. Approval was based on a randomized, placebo-controlled, phase III trial that showed a marked progression-free survival (PFS) improvement for patients randomly assigned to receive vandetanib (hazard ratio, 0.35); the trial also showed an objective response rate advantage for patients receiving vandetanib (44% vs. 1% for the placebo arm).[
4
][
5
]
Children with locally advanced or metastatic medullary thyroid carcinoma were treated with vandetanib in a phase I/II trial. Of 16 patients, only 1 had no response, and 7 had a partial response, for an objective response rate of 44%. Disease in three of those patients subsequently recurred, but 11 of 16 patients treated with vandetanib remained on therapy at the time of the report. The median duration of therapy for the entire cohort was 27 months, with a range of 2 to 52 months.[ 6 ] A long-term outcome evaluation in a cohort of 17 children and adolescents with advanced medullary thyroid carcinoma who received vandetanib reported a median PFS of 6.7 years and a 5-year overall survival of 88.2%.[ 7 ]
- Cabozantinib. Cabozantinib (an inhibitor of the RET and MET kinases and VEGFR) has also shown activity against unresectable medullary thyroid cancer (10 of 35 adult patients [29%] had a partial response).[ 8 ] Cabozantinib was approved by the FDA in November 2012 for the treatment of adults with metastatic medullary thyroid cancer.
-
Vandetanib. Vandetanib (an inhibitor of RET kinase, vascular endothelial growth factor receptor [VEGFR], and epidermal growth factor receptor signaling) is approved by the U.S. Food and Drug Administration (FDA) for the treatment of symptomatic or progressive medullary thyroid cancer in adult patients with unresectable, locally advanced, or metastatic disease. Approval was based on a randomized, placebo-controlled, phase III trial that showed a marked progression-free survival (PFS) improvement for patients randomly assigned to receive vandetanib (hazard ratio, 0.35); the trial also showed an objective response rate advantage for patients receiving vandetanib (44% vs. 1% for the placebo arm).[
4
][
5
]
(Refer to the PDQ summary on Childhood Multiple Endocrine Neoplasia [MEN] Syndromes Treatment and the Treatment for Medullary Thyroid Cancer [MTC] section in the PDQ summary on Genetics of Endocrine and Neuroendocrine Neoplasias for more information.)
Treatment Options Under Clinical Evaluation for Medullary Thyroid Carcinoma
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
-
APEC1621 (NCT03155620) (Pediatric MATCH: Targeted Therapy Directed by Genetic Testing in Treating Pediatric Patients with Relapsed or Refractory Advanced Solid Tumors, Non-Hodgkin Lymphomas, or Histiocytic Disorders): NCI-COG Pediatric Molecular Analysis for Therapeutic Choice (MATCH), referred to as Pediatric MATCH, will match targeted agents with specific molecular changes identified using a next-generation sequencing targeted assay of more than 4,000 different mutations across more than 160 genes in refractory and recurrent solid tumors. Children and adolescents aged 1 to 21 years are eligible for the trial.
Tumor tissue from progressive or recurrent disease must be available for molecular characterization. Patients with tumors that have molecular variants addressed by treatment arms included in the trial will be offered treatment on Pediatric MATCH. Additional information can be obtained on the NCI website and ClinicalTrials.gov website.
参考文献- Machens A, Elwerr M, Thanh PN, et al.: Impact of central node dissection on postoperative morbidity in pediatric patients with suspected or proven thyroid cancer. Surgery 160 (2): 484-92, 2016.[PUBMED Abstract]
- Wells SA, Asa SL, Dralle H, et al.: Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid 25 (6): 567-610, 2015.[PUBMED Abstract]
- Machens A, Elwerr M, Lorenz K, et al.: Long-term outcome of prophylactic thyroidectomy in children carrying RET germline mutations. Br J Surg 105 (2): e150-e157, 2018.[PUBMED Abstract]
- Wells SA, Robinson BG, Gagel RF, et al.: Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 30 (2): 134-41, 2012.[PUBMED Abstract]
- Thornton K, Kim G, Maher VE, et al.: Vandetanib for the treatment of symptomatic or progressive medullary thyroid cancer in patients with unresectable locally advanced or metastatic disease: U.S. Food and Drug Administration drug approval summary. Clin Cancer Res 18 (14): 3722-30, 2012.[PUBMED Abstract]
- Fox E, Widemann BC, Chuk MK, et al.: Vandetanib in children and adolescents with multiple endocrine neoplasia type 2B associated medullary thyroid carcinoma. Clin Cancer Res 19 (15): 4239-48, 2013.[PUBMED Abstract]
- Kraft IL, Akshintala S, Zhu Y, et al.: Outcomes of Children and Adolescents with Advanced Hereditary Medullary Thyroid Carcinoma Treated with Vandetanib. Clin Cancer Res 24 (4): 753-765, 2018.[PUBMED Abstract]
- Kurzrock R, Sherman SI, Ball DW, et al.: Activity of XL184 (Cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 29 (19): 2660-6, 2011.[PUBMED Abstract]
-
Surgery: Treatment for children with medullary thyroid carcinoma is mainly surgical. Investigators have concluded that prophylactic central node dissection should not be performed on patients with hereditary medullary thyroid cancer if their basal calcitonin serum levels are lower than 40 pg/mL.[
1
]
- Special Considerations for the Treatment of Children With Cancer
-
Cancer in children and adolescents is rare, although the overall incidence of childhood cancer has been slowly increasing since 1975.[ 1 ] Referral to medical centers with multidisciplinary teams of cancer specialists experienced in treating cancers that occur in childhood and adolescence should be considered for children and adolescents with cancer. This multidisciplinary team approach incorporates the skills of the following health care professionals and others to ensure that children receive treatment, supportive care, and rehabilitation that will achieve optimal survival and quality of life:
- Primary care physicians.
- Pediatric surgeons.
- Radiation oncologists.
- Pediatric medical oncologists/hematologists.
- Rehabilitation specialists.
- Pediatric nurse specialists.
- Social workers.
- Child-life professionals.
- Psychologists.
(Refer to the PDQ Supportive and Palliative Care summaries for specific information about supportive care for children and adolescents with cancer.)
Guidelines for pediatric cancer centers and their role in the treatment of pediatric patients with cancer have been outlined by the American Academy of Pediatrics.[ 2 ] At these pediatric cancer centers, clinical trials are available for most types of cancer that occur in children and adolescents, and the opportunity to participate in these trials is offered to most patients and their families. Clinical trials for children and adolescents diagnosed with cancer are generally designed to compare potentially better therapy with therapy that is currently accepted as standard. Most of the progress made in identifying curative therapy for childhood cancers has been achieved through clinical trials. Information about ongoing clinical trials is available from the NCI website.
Dramatic improvements in survival have been achieved for children and adolescents with cancer. Between 1975 and 2010, childhood cancer mortality decreased by more than 50%.[ 3 ] Childhood and adolescent cancer survivors require close monitoring because cancer therapy side effects may persist or develop months or years after treatment. (Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors.)
Childhood cancer is a rare disease, with about 15,000 cases diagnosed annually in the United States in individuals younger than 20 years.[ 4 ] The U.S. Rare Diseases Act of 2002 defines a rare disease as one that affects populations smaller than 200,000 persons. Therefore, all pediatric cancers are considered rare.
The designation of a rare tumor is not uniform among pediatric and adult groups. Adult rare cancers are defined as those with an annual incidence of fewer than six cases per 100,000 people, and they are estimated to account for up to 24% of all cancers diagnosed in the European Union and about 20% of all cancers diagnosed in the United States.[ 5 ][ 6 ] Also, the designation of a pediatric rare tumor is not uniform among international groups, as follows:
- The Italian cooperative project on rare pediatric tumors (Tumori Rari in Eta Pediatrica [TREP]) defines a pediatric rare tumor as one with an incidence of less than two cases per 1 million population per year and is not included in other clinical trials.[ 7 ]
- The Children's Oncology Group has opted to define rare pediatric cancers as those listed in the International Classification of Childhood Cancer subgroup XI, which includes thyroid cancer, melanoma and nonmelanoma skin cancers, and multiple types of carcinomas (e.g., adrenocortical carcinoma, nasopharyngeal carcinoma, and most adult-type carcinomas such as breast cancer, colorectal cancer, etc.).[
8
] These diagnoses account for about 4% of cancers diagnosed in children aged 0 to 14 years, compared with about 20% of cancers diagnosed in adolescents aged 15 to 19 years.[
9
]
Most cancers within subgroup XI are either melanomas or thyroid cancer, with the remaining subgroup XI cancer types accounting for only 1.3% of cancers in children aged 0 to 14 years and 5.3% of cancers in adolescents aged 15 to 19 years.
These rare cancers are extremely challenging to study because of the low incidence of patients with any individual diagnosis, the predominance of rare cancers in the adolescent population, and the lack of clinical trials for adolescents with rare cancers.
Information about these tumors may also be found in sources relevant to adults with cancer such as the PDQ summary on Thyroid Cancer Treatment (Adult).
参考文献- Smith MA, Seibel NL, Altekruse SF, et al.: Outcomes for children and adolescents with cancer: challenges for the twenty-first century. J Clin Oncol 28 (15): 2625-34, 2010.[PUBMED Abstract]
- Corrigan JJ, Feig SA; American Academy of Pediatrics: Guidelines for pediatric cancer centers. Pediatrics 113 (6): 1833-5, 2004.[PUBMED Abstract]
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014.[PUBMED Abstract]
- Ward E, DeSantis C, Robbins A, et al.: Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin 64 (2): 83-103, 2014 Mar-Apr.[PUBMED Abstract]
- Gatta G, Capocaccia R, Botta L, et al.: Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet-a population-based study. Lancet Oncol 18 (8): 1022-1039, 2017.[PUBMED Abstract]
- DeSantis CE, Kramer JL, Jemal A: The burden of rare cancers in the United States. CA Cancer J Clin 67 (4): 261-272, 2017.[PUBMED Abstract]
- Ferrari A, Bisogno G, De Salvo GL, et al.: The challenge of very rare tumours in childhood: the Italian TREP project. Eur J Cancer 43 (4): 654-9, 2007.[PUBMED Abstract]
- Pappo AS, Krailo M, Chen Z, et al.: Infrequent tumor initiative of the Children's Oncology Group: initial lessons learned and their impact on future plans. J Clin Oncol 28 (33): 5011-6, 2010.[PUBMED Abstract]
- Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2012. Bethesda, Md: National Cancer Institute, 2015. Also available online. Last accessed February 20, 2020.[PUBMED Abstract]
- Changes to This Summary (06/08/2020)
-
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Clinical Presentation and Prognostic Factors
Added text about the results of a German Society for Pediatric Oncology and Hematology–Malignant Endocrine Tumors registry study that identified a total of 57 patients with medullary thyroid carcinoma and 17 patients with C-cell hyperplasia (cited Kuhlen et al. as reference 12 and level of evidence 3iA).
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
- About This PDQ Summary
-
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of childhood thyroid cancer. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
- Denise Adams, MD (Children's Hospital Boston)
- Karen J. Marcus, MD, FACR (Dana-Farber Cancer Institute/Boston Children's Hospital)
- Paul A. Meyers, MD (Memorial Sloan-Kettering Cancer Center)
- Thomas A. Olson, MD (Aflac Cancer and Blood Disorders Center of Children's Healthcare of Atlanta - Egleston Campus)
- Alberto S. Pappo, MD (St. Jude Children's Research Hospital)
- Arthur Kim Ritchey, MD (Children's Hospital of Pittsburgh of UPMC)
- Carlos Rodriguez-Galindo, MD (St. Jude Children's Research Hospital)
- Stephen J. Shochat, MD (St. Jude Children's Research Hospital)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Childhood Thyroid Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/thyroid/hp/child-thyroid-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389315]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.

 画像を拡大する
画像を拡大する