ご利用について
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of retinoblastoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
CONTENTS
- General Information About Retinoblastoma
-
Retinoblastoma is a pediatric cancer that requires careful integration of multidisciplinary care. Treatment of retinoblastoma aims to save the patient's life and uses an individualized, risk-adapted approach to minimize systemic exposure to drugs, optimize ocular drug delivery, and preserve useful vision. For patients presenting with extraocular retinoblastoma, treatment with systemic chemotherapy and radiation therapy is likely to be curative. However, extraorbital disease requires intensive chemotherapy and may include consolidation with high-dose chemotherapy and autologous hematopoietic stem cell rescue with or without radiation therapy. While a large proportion of patients with systemic extra–central nervous system (CNS) metastases can be cured, the prognosis for patients with intracranial disease is dismal.
Incidence
Retinoblastoma is a relatively uncommon tumor of childhood that arises in the retina and accounts for about 3% of the cancers occurring in children younger than 15 years.
Retinoblastoma is a cancer of the very young child; two-thirds of all cases of retinoblastoma are diagnosed before age 2 years.[ 1 ] Thus, while the estimated annual incidence in the United States is approximately 4 cases per 1 million children younger than 15 years, the age-adjusted annual incidence in children aged 0 to 4 years is 10 to 14 cases per 1 million (approximately 1 in 14,000–18,000 live births).
Anatomy
Retinoblastoma arises from the retina, and it may grow under the retina and/or toward the vitreous cavity. Involvement of the ocular coats and optic nerve occurs as a sequence of events as the tumor progresses.
Focal invasion of the choroid is common, although the occurrence of massive invasion is usually limited to advanced disease. After invading the choroid, the tumor gains access to systemic circulation and creates the potential for metastases. Further progression through the ocular coats leads to invasion of the sclera and the orbit. Tumors that invade the anterior chamber may gain access to systemic circulation through the canal of Schlemm. Progression through the optic nerve and past the lamina cribrosa increases the risk of systemic and CNS dissemination (refer to Figure 1).
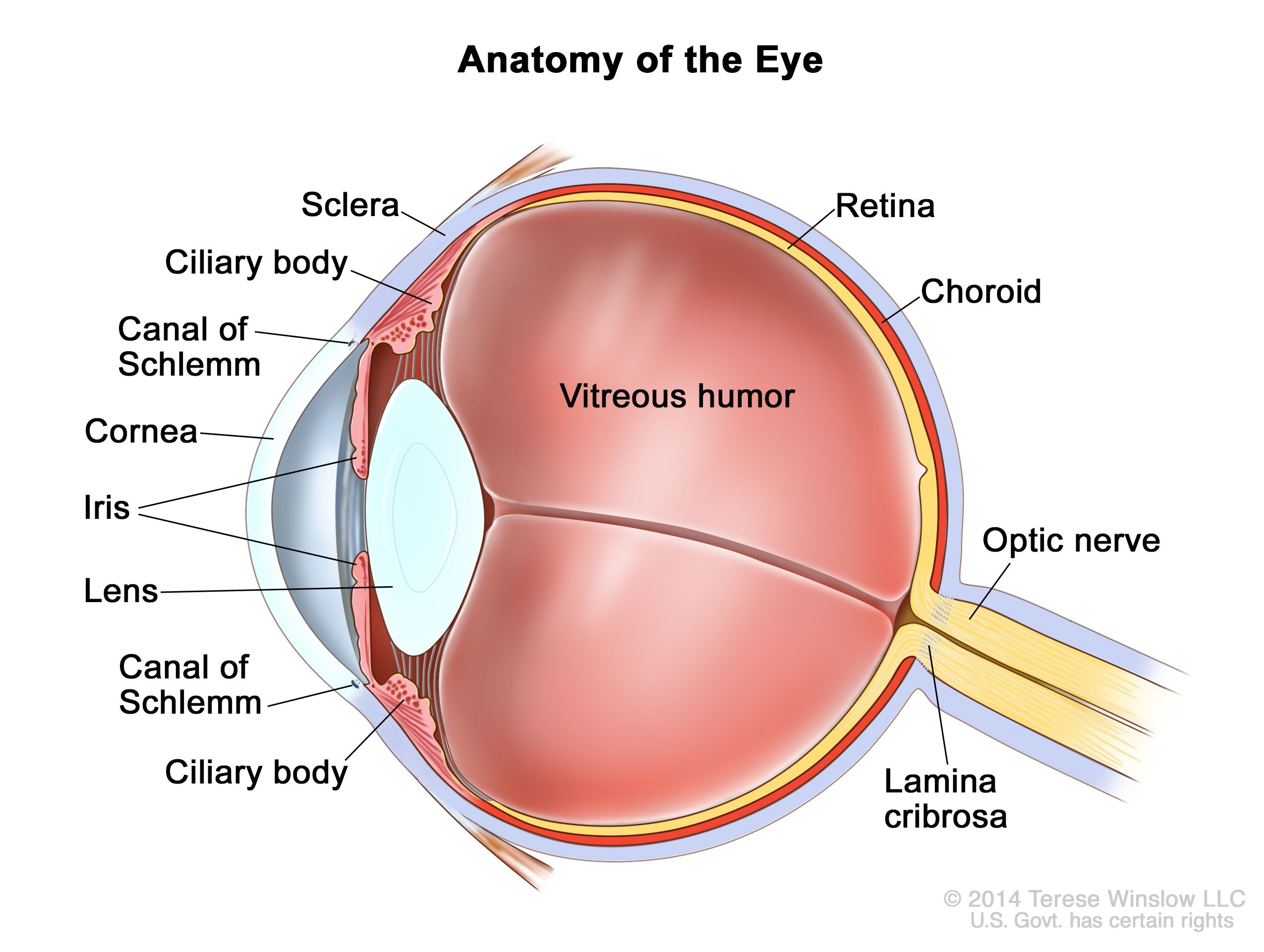
Figure 1. Anatomy of the eye showing the sclera, ciliary body, canal of Schlemm, cornea, iris, lens, vitreous humor, retina, choroid, optic nerve, and lamina cribrosa. The vitreous humor is a gel that fills the center of the eye. Screening
Recent consensus reports from the American Association of Ophthalmic Oncologists and Pathologists and the American Association for Cancer Research Childhood Cancer Predisposition Workshop describe surveillance guidelines for screening children at risk of developing retinoblastoma.[ 2 ][ 3 ]
In children with a positive family history of retinoblastoma, early-in-life screening by fundus exam is performed under general anesthesia at regular intervals according to a schedule based on the absolute estimated risk, as determined by the identification of the RB1 mutation in the family and the presence of the RB1 mutation in the child.[ 2 ][ 3 ]
Infants born to affected parents have a dilated eye examination under anesthesia as soon as possible in the first month of life, and a genetic evaluation is performed. Infants with a positive genetic test are examined under anesthesia on a monthly basis. In infants who do not develop disease, monthly exams continue throughout the first year; the frequency of those studies may be decreased progressively during the second and subsequent years. Screening exams can improve prognosis in terms of globe sparing and use of less intensive, ocular-salvage treatments in children with a positive family history of retinoblastoma (refer to Table 1 and Figure 2).[ 2 ][ 3 ]
Table 1. Pretest Risk for Relatives to Carry the Mutant RB1 Allele of the Probanda,b Relative of Proband Pretest Risk for Mutant Allele (%) Bilateral Proband (100) Unilateral Proband (15) aReprinted from Ophthalmology, Volume 125, Issue 3, Alison H. Skalet, Dan S. Gombos, Brenda L. Gallie, Jonathan W. Kim, Carol L. Shields, Brian P. Marr, Sharon E. Plon, Patricia Chévez-Barrios, Screening Children at Risk for Retinoblastoma: Consensus Report from the American Association of Ophthalmic Oncologists and Pathologists, Pages 453–458, Copyright (2018), with permission from Elsevier. bPretest risk for RB1 mutation in family members of an affected child with retinoblastoma. Risk for RB1 mutant allele is shown as a percentage for unilateral and bilateral probands without family history of retinoblastoma. cThird- and fourth-degree relatives of unilateral probands have calculated risks of 0.003% and 0.001%, respectively, which are less than the normal population risk of 0.007% (1 in 15,000 live births); therefore, the risk is stated at 0.007%. Offspring (infant) 50 7.5 Parent 5 0.8 Sibling 2.5 0.4 Niece/nephew 1.3 0.2 Aunt/uncle 0.1 0.007c First cousin 0.05 0.007c General population 0.007 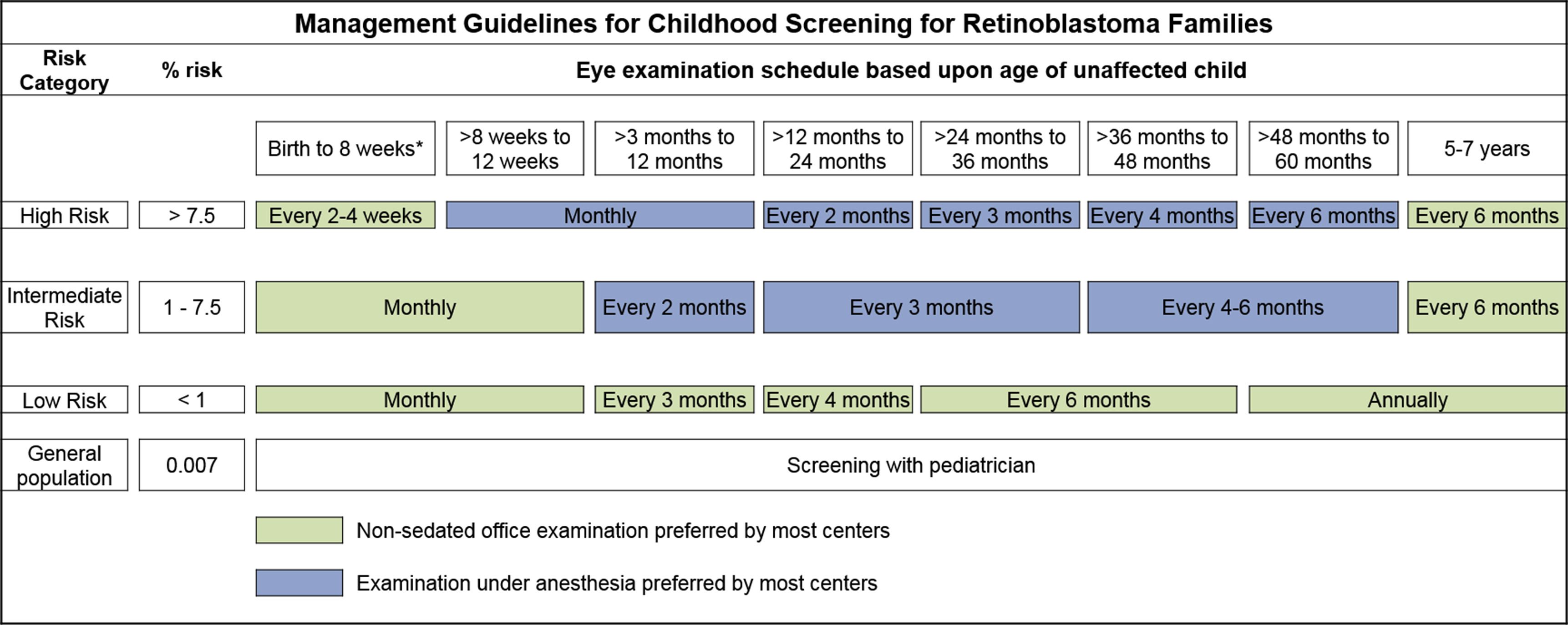
Figure 2. Management guidelines for childhood screening for retinoblastoma. The presented schedules are general guidelines and reflect a schedule for examinations in which no lesions of concern are noted. It may be appropriate to examine some children more frequently. Decisions regarding examination method, examination under anesthesia (EUA) versus nonsedated examination in the office, are complex and best decided by the clinician in discussion with the patient's family. The preference of the majority of the clinical centers involved in the creation of this consensus statement is reflected, but individual centers may make policy decisions based on available resources and expert clinician preference. Examination under anesthesia will be strongly considered for any child who is unable to participate in an office examination sufficiently to allow thorough examination of the retina. *A minority of clinical centers also prefer EUA for high- and intermediate-risk children (calculated risk >1%) from birth to 8 weeks of age. Reprinted from Ophthalmology, Volume 125, Issue 3, Alison H. Skalet, Dan S. Gombos, Brenda L. Gallie, Jonathan W. Kim, Carol L. Shields, Brian P. Marr, Sharon E. Plon, Patricia Chévez-Barrios, Screening Children at Risk for Retinoblastoma: Consensus Report from the American Association of Ophthalmic Oncologists and Pathologists, Pages 453–458, Copyright (2018), with permission from Elsevier. It is common practice to use ophthalmic examinations to screen the parents and siblings of patients with retinoblastoma to exclude an unknown familial disease. Controversy exists regarding screening a child born to a parent who had unilateral retinoblastoma, but who has not had genetic testing.[ 4 ] This debate underscores the importance of genetic testing. In the future, if all patients with retinoblastoma undergo genetic testing, the assessment of risk to their offspring can be determined rather than estimated.
Clinical Presentation
Age at presentation correlates with laterality; patients with bilateral disease present at a younger age, usually in the first 12 months of life.
Most patients present with leukocoria, which is occasionally first noticed after a flash photograph is taken. Strabismus is the second most common presenting sign and usually correlates with macular involvement. Very advanced intraocular tumors present with pain, orbital cellulitis, glaucoma, or buphthalmos.
As the tumor progresses, patients may present with orbital or metastatic disease. Metastases occur in the preauricular and laterocervical lymph nodes, in the CNS, or systemically (commonly in the bones, bone marrow, and liver).
In the United States, children of Hispanic origin and children living in lower socioeconomic conditions have been noted to present with more advanced disease.[ 5 ]
Diagnostic and Staging Evaluation
Diagnostic evaluation of retinoblastoma includes the following:
- Eye examination. Intraocular retinoblastoma is usually diagnosed without pathologic confirmation. An examination under anesthesia with a maximally dilated pupil and scleral indentation is required to examine the entire retina. A very detailed documentation of the number, location, and size of tumors; the presence of retinal detachment and subretinal fluid; and the presence of subretinal and vitreous seeds must be performed.
- Ocular ultrasound and magnetic resonance imaging (MRI). Bidimensional ocular ultrasound and MRI can be useful to differentiate retinoblastoma from other causes of leukocoria and in the evaluation of extrascleral and extraocular extension in children with advanced intraocular retinoblastoma. Optic nerve enhancement by MRI does not necessarily indicate involvement; cautious interpretation of those findings is needed.[ 6 ]
- Reverse transcriptase–polymerase chain reaction (RT-PCR). The detection of the synthetase of ganglioside GD2 mRNA by RT-PCR in the cerebrospinal fluid at the time of diagnosis may be a marker for CNS disease.[ 7 ]
Evaluation for the presence of metastatic disease also needs to be considered in the subgroup of patients with suspected extraocular extension by imaging or high-risk pathology in the enucleated eye (i.e., massive choroidal invasion or involvement of the sclera or the optic nerve beyond the lamina cribrosa). Patients presenting with these pathological features in the enucleated eye are at high risk of developing metastases. In these cases, the following procedures may be performed:[ 8 ]
Heritable and Nonheritable Forms of Retinoblastoma
Retinoblastoma is a tumor that occurs in heritable (25%–30%) and nonheritable (70%–75%) forms. Heritable disease is defined by the presence of a germline mutation of the RB1 gene. This germline mutation may have been inherited from an affected progenitor (25% of cases) or may have occurred in a germ cell before conception or in utero during early embryogenesis in patients with sporadic disease (75% of cases). The presence of positive family history or bilateral or multifocal disease is suggestive of heritable disease.
Heritable retinoblastoma may manifest as unilateral or bilateral disease. The penetrance of the RB1 mutation (laterality, age at diagnosis, and number of tumors) is probably dependent on concurrent genetic modifiers such as MDM2 and MDM4 polymorphisms.[ 9 ][ 10 ] All children with bilateral disease and approximately 15% of patients with unilateral disease are presumed to have the heritable form, even though only 25% have an affected parent.
Children with heritable retinoblastoma tend to be diagnosed at a younger age than are children with the nonheritable form of the disease. It was thought that unilateral retinoblastoma in children younger than 1 year raises concern for the presence of heritable disease, whereas older children with a unilateral tumor are more likely to have the nonheritable form of the disease.[ 11 ] However, in a retrospective single-institution report of 182 patients with unilateral retinoblastoma, patients with a positive genetic result (n = 32) were diagnosed at a mean age of 26 months, and patients without genetic results were diagnosed at a mean age of 22 months (P = .31).[ 12 ]
The genomic landscape of retinoblastoma is driven by alterations in RB1 that lead to biallelic inactivation.[ 13 ][ 14 ] A rare cause of RB1 inactivation is chromothripsis, which may be difficult to detect by conventional methods.[ 15 ]
Other recurring genomic changes that occur in a small minority of tumors include BCOR mutation/deletion, MYCN amplification, and OTX2 amplification.[ 13 ][ 14 ][ 15 ] A study of 1,068 unilateral nonfamilial retinoblastoma tumors reported that a small percentage of cases (approximately 3%) lacked evidence of RB1 loss. Approximately one-half of these cases with no evidence of RB1 loss (representing approximately 1.5% of all unilateral nonfamilial retinoblastoma) showed MYCN amplification.[ 14 ] The functional status of the retinoblastoma protein (pRb) is inferred to be inactive in retinoblastoma with MYCN amplification. This suggests that inactivation of RB1 by mutation or inactive pRb is a requirement for the development of retinoblastoma, independent of MYCN amplification.[ 16 ]
Genetic counseling is recommended for all patients with retinoblastoma. (Refer to the Genetic Counseling section of the PDQ summary on Retinoblastoma Treatment for more information.)
Genetic Testing
Blood and tumor samples can be tested to determine whether a patient with retinoblastoma has a germline or somatic mutation in the RB1 gene. Once the patient's genetic mutation has been identified, other family members can be screened directly for the mutation with targeted sequencing.
A multistep assay that includes the following may be performed for a complete genetic evaluation of the RB1 gene:[ 17 ]
In cases of somatic mosaicism or cytogenetic abnormalities, the mutations may not be easily detected; more exhaustive techniques such as karyotyping, fluorescence in situ hybridization, and methylation analysis of the RB1 promoter may be needed. Deep (2500x) sequencing of an RB1 genomic amplicon from lymphocyte DNA can reveal low-level mosaicism.[ 18 ] Because mosaicism is caused by a postzygotic mutation, such a finding obviates the need for serial examination of siblings under anesthesia. Current technologies will not discover some mosaic mutations at very low levels of amplification, mutations outside of the RB1 coding exons or the flanking intronic regions, mutations not found in lymphocytes but in other tissues (mosaic), or mosaic large rearrangements of RB1.[ 18 ] Combining the above techniques, a germline mutation may be detected in more than 90% of patients with heritable retinoblastoma.[ 19 ][ 20 ][ 21 ]
The absence of detectable somatic RB1 mutations in approximately 3% of unilateral, nonheritable retinoblastoma cases suggests that alternative genetic mechanisms may underlie the development of retinoblastoma.[ 22 ] In one-half of these cases, high levels of MYCN amplification have been reported; these patients had distinct, aggressive histologic features and a median age at diagnosis of 4 months.[ 14 ] However, MYCN amplification has also been reported to coexist with RB1 mutations.[ 16 ] In another small subset of tumors without detectable somatic RB1 mutations, chromothripsis is responsible for inactivating the RB1 gene.[ 15 ]
Genetic Counseling
Genetic counseling is an integral part of the management of patients with retinoblastoma and their families, regardless of clinical presentation. Counseling includes a discussion of the main forms of retinoblastoma, which assists parents in understanding the genetic consequences of each form of retinoblastoma and in estimating the risk of disease in family members.[ 19 ] Counseling also includes guidance towards appropriate screening for both patients and their families, especially if the risk of developing a second primary malignancy is increased.
Genetic counseling, however, is not always straightforward. Approximately 10% of children with retinoblastoma have somatic genetic mosaicism, which contributes to the difficulty of genetic counseling.[ 23 ] In addition, for one specific mutation, the risk of retinoblastoma in a sibling may depend partly on whether the mutation is inherited from the mother or father.[ 24 ] (Refer to the PDQ summary on Cancer Genetics Risk Assessment and Counseling for more information.)
Postdiagnosis Surveillance
Children with a germline RB1 mutation may continue to develop new tumors for a few years after diagnosis and treatment; for this reason, they need to be examined frequently. It is common practice for examinations to occur every 2 to 4 months for at least 28 months.[ 25 ] The interval between exams is based on the stability of the disease and age of the child (i.e., less frequent visits as the child ages).
A proportion of children who present with unilateral retinoblastoma will eventually develop disease in the opposite eye. Periodic examinations of the unaffected eye are performed until the germline status of the RB1 gene is determined.
Because of the poor prognosis for patients with trilateral retinoblastoma, screening with neuroimaging until age 5 years is a common practice in the monitoring of children with the heritable form of the disease. (Refer to the Trilateral retinoblastoma section in the Causes of Retinoblastoma-Related Mortality section of this summary for more information.)
Causes of Retinoblastoma-Related Mortality
While retinoblastoma is a highly curable disease, the challenge for those who treat retinoblastoma is to preserve life and to prevent the loss of an eye, blindness, and other serious effects of treatment that reduce the patient's life span or quality of life. With improvements in the diagnosis and management of retinoblastoma over the past several decades, metastatic retinoblastoma is observed less frequently in the United States and other developed nations. As a result, other causes, such as trilateral retinoblastoma and subsequent neoplasms (SNs), have become significant contributors to retinoblastoma-related mortality in the first and subsequent decades of life.
In the United States, before the advent of chemoreduction as a means of treating heritable or bilateral disease and the implementation of neuroimaging screening, trilateral retinoblastoma contributed to more than 50% of retinoblastoma-related mortality in the first decade after diagnosis.[ 26 ] Second neoplasms are the most common cause of death and contribute to about 50% of deaths in patients with bilateral disease and genetically defined heritable retinoblastoma.[ 27 ][ 28 ][ 29 ]
Trilateral retinoblastoma
Trilateral retinoblastoma is a well-recognized syndrome that occurs in 5% to 15% of patients with heritable retinoblastoma. It is defined by the development of an intracranial midline neuroblastic tumor, which typically develops between the ages of 20 and 36 months.[ 30 ]
Trilateral retinoblastoma has been the principal cause of death from retinoblastoma in the United States during the first decade of life.[ 31 ] Because of the poor prognosis for patients with trilateral retinoblastoma and the apparent improved survival with early detection and aggressive treatment, screening with routine neuroimaging could potentially detect most cases within 2 years of first diagnosis.[ 30 ] Routine baseline brain MRI is recommended at diagnosis because it may detect trilateral retinoblastoma at a subclinical stage. In a small series, the 5-year overall survival rate was 67% for patients with tumors that were detected at baseline, compared with 11% for the group with a delayed diagnosis.[ 32 ]
Although it is not clear whether early diagnosis can impact survival, screening with MRI has been recommended as often as every 6 months for 5 years for patients suspected of having heritable disease or those with unilateral disease and a positive family history.[ 33 ] Computed tomography scans are generally avoided for routine screening in these children because of the risk related to ionizing radiation exposure.
A cystic pineal gland, which is commonly detected by surveillance MRI, needs to be distinguished from a cystic variant of pineoblastoma. In children without retinoblastoma, the incidence of pineal cysts has been reported to be 55.8%.[ 34 ] In a case-control study that included 77 children with retinoblastoma and 77 controls, the incidence of pineal cysts was similar (61% and 69%, respectively), and the size and volume of the pineal gland was not significantly different between the groups.[ 35 ] However, a cystic component has been described in up to 57% of patients with histologically confirmed trilateral retinoblastoma.[ 32 ] An excessive increase in the size of the pineal gland seems to be the strongest parameter indicating a malignant process.[ 35 ]
Subsequent neoplasms (SNs)
Survivors of retinoblastoma have a high risk of developing SNs.
Factors that influence the risk of SNs include the following:
The issue of balancing long-term tumor control with the consequences of chemotherapy is unresolved. Most patients who receive chemotherapy are exposed to etoposide, which has been associated with secondary leukemia in patients without a predisposition to cancer, but at modest rates when compared with the risks associated with EBRT in heritable retinoblastoma.
Despite the known increased risk of acute myeloid leukemia (AML) associated with the use of etoposide, patients with heritable retinoblastoma are not at an increased risk of developing this SN.[ 50 ][ 51 ][ 52 ] An initial report conducted by informal survey methods described 15 patients who developed AML after chemotherapy. One-half of the patients also received radiation therapy.[ 51 ] This finding has not been substantiated by formal studies. In a single-institution study of 245 patients who received etoposide, only 1 patient had acute promyelocytic leukemia after 79 months.[ 50 ] Additionally, the Surveillance, Epidemiology, and End Results (SEER) program calculated standardized incidence rates for secondary hematopoietic malignancies in 34,867 survivors of childhood cancer. The observed-to-expected ratio of secondary AML in patients treated for retinoblastoma was zero.[ 53 ]
Survival from SNs is certainly suboptimal and varies widely across studies.[ 38 ][ 54 ][ 55 ][ 56 ][ 57 ][ 58 ] However, with advances in therapy, it is essential that all SNs in survivors of retinoblastoma be treated with curative intent.[ 59 ]
Late Effects From Retinoblastoma Therapy
In a report from the Retinoblastoma Survivor Study (N = 470), 87% of survivors of retinoblastoma (mean age, 43 years; median follow-up, 42 years) had at least one medical condition and 71% had a severe or life-threatening condition. The adjusted relative risk of a chronic condition in survivors, compared with nonretinoblastoma controls, was 1.4 (P < .01); the relative risk of a grade 3 or 4 condition was 7.6 (P < .01). After excluding ocular conditions and SNs, this excess risk was found to persist only for patients with bilateral disease.[ 60 ]
As previously discussed, patients with heritable retinoblastoma have an increased incidence of SNs. (Refer to the Subsequent neoplasms [SNs] section of this summary for more information.) Other late effects that may occur after treatment for retinoblastoma include the following:
Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors.
参考文献- Ries LA, Smith MA, Gurney JG, et al., eds.: Cancer incidence and survival among children and adolescents: United States SEER Program 1975-1995. Bethesda, Md: National Cancer Institute, SEER Program, 1999. NIH Pub.No. 99-4649. Also available online. Last accessed August 09, 2019.[PUBMED Abstract]
- Skalet AH, Gombos DS, Gallie BL, et al.: Screening Children at Risk for Retinoblastoma: Consensus Report from the American Association of Ophthalmic Oncologists and Pathologists. Ophthalmology 125 (3): 453-458, 2018.[PUBMED Abstract]
- Kamihara J, Bourdeaut F, Foulkes WD, et al.: Retinoblastoma and Neuroblastoma Predisposition and Surveillance. Clin Cancer Res 23 (13): e98-e106, 2017.[PUBMED Abstract]
- Abramson DH: Re: Skalet et al.: Screening children at risk for retinoblastoma: consensus report from the American Association of Ophthalmic Oncologists and Pathologists (Ophthalmology. 2018;125:453-458). Ophthalmology 125 (9): e63-e64, 2018.[PUBMED Abstract]
- Truong B, Green AL, Friedrich P, et al.: Ethnic, Racial, and Socioeconomic Disparities in Retinoblastoma. JAMA Pediatr 169 (12): 1096-104, 2015.[PUBMED Abstract]
- Khurana A, Eisenhut CA, Wan W, et al.: Comparison of the diagnostic value of MR imaging and ophthalmoscopy for the staging of retinoblastoma. Eur Radiol 23 (5): 1271-80, 2013.[PUBMED Abstract]
- Laurent VE, Sampor C, Solernou V, et al.: Detection of minimally disseminated disease in the cerebrospinal fluid of children with high-risk retinoblastoma by reverse transcriptase-polymerase chain reaction for GD2 synthase mRNA. Eur J Cancer 49 (13): 2892-9, 2013.[PUBMED Abstract]
- Kaliki S, Shields CL, Rojanaporn D, et al.: High-risk retinoblastoma based on international classification of retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology 120 (5): 997-1003, 2013.[PUBMED Abstract]
- Castéra L, Sabbagh A, Dehainault C, et al.: MDM2 as a modifier gene in retinoblastoma. J Natl Cancer Inst 102 (23): 1805-8, 2010.[PUBMED Abstract]
- de Oliveira Reis AH, de Carvalho IN, de Sousa Damasceno PB, et al.: Influence of MDM2 and MDM4 on development and survival in hereditary retinoblastoma. Pediatr Blood Cancer 59 (1): 39-43, 2012.[PUBMED Abstract]
- Zajaczek S, Jakubowska A, Kurzawski G, et al.: Age at diagnosis to discriminate those patients for whom constitutional DNA sequencing is appropriate in sporadic unilateral retinoblastoma. Eur J Cancer 34 (12): 1919-21, 1998.[PUBMED Abstract]
- Berry JL, Lewis L, Zolfaghari E, et al.: Lack of correlation between age at diagnosis and RB1 mutations for unilateral retinoblastoma: the importance of genetic testing. Ophthalmic Genet 39 (3): 407-409, 2018.[PUBMED Abstract]
- Zhang J, Benavente CA, McEvoy J, et al.: A novel retinoblastoma therapy from genomic and epigenetic analyses. Nature 481 (7381): 329-34, 2012.[PUBMED Abstract]
- Rushlow DE, Mol BM, Kennett JY, et al.: Characterisation of retinoblastomas without RB1 mutations: genomic, gene expression, and clinical studies. Lancet Oncol 14 (4): 327-34, 2013.[PUBMED Abstract]
- McEvoy J, Nagahawatte P, Finkelstein D, et al.: RB1 gene inactivation by chromothripsis in human retinoblastoma. Oncotarget 5 (2): 438-50, 2014.[PUBMED Abstract]
- Ewens KG, Bhatti TR, Moran KA, et al.: Phosphorylation of pRb: mechanism for RB pathway inactivation in MYCN-amplified retinoblastoma. Cancer Med 6 (3): 619-630, 2017.[PUBMED Abstract]
- Clark R: Retinoblastoma: genetic testing and counseling. In: Singh A, Damato B: Clinical Ophthalmic Oncology. Philadelphia, Pa: Saunders Elsevier, 2007, pp 441-6.[PUBMED Abstract]
- Amitrano S, Marozza A, Somma S, et al.: Next generation sequencing in sporadic retinoblastoma patients reveals somatic mosaicism. Eur J Hum Genet 23 (11): 1523-30, 2015.[PUBMED Abstract]
- Richter S, Vandezande K, Chen N, et al.: Sensitive and efficient detection of RB1 gene mutations enhances care for families with retinoblastoma. Am J Hum Genet 72 (2): 253-69, 2003.[PUBMED Abstract]
- Sagi M, Frenkel A, Eilat A, et al.: Genetic screening in patients with Retinoblastoma in Israel. Fam Cancer 14 (3): 471-80, 2015.[PUBMED Abstract]
- Chen Z, Moran K, Richards-Yutz J, et al.: Enhanced sensitivity for detection of low-level germline mosaic RB1 mutations in sporadic retinoblastoma cases using deep semiconductor sequencing. Hum Mutat 35 (3): 384-91, 2014.[PUBMED Abstract]
- Nichols KE, Houseknecht MD, Godmilow L, et al.: Sensitive multistep clinical molecular screening of 180 unrelated individuals with retinoblastoma detects 36 novel mutations in the RB1 gene. Hum Mutat 25 (6): 566-74, 2005.[PUBMED Abstract]
- Dommering CJ, Mol BM, Moll AC, et al.: RB1 mutation spectrum in a comprehensive nationwide cohort of retinoblastoma patients. J Med Genet 51 (6): 366-74, 2014.[PUBMED Abstract]
- Eloy P, Dehainault C, Sefta M, et al.: A Parent-of-Origin Effect Impacts the Phenotype in Low Penetrance Retinoblastoma Families Segregating the c.1981C>T/p.Arg661Trp Mutation of RB1. PLoS Genet 12 (2): e1005888, 2016.[PUBMED Abstract]
- Abramson DH, Mendelsohn ME, Servodidio CA, et al.: Familial retinoblastoma: where and when? Acta Ophthalmol Scand 76 (3): 334-8, 1998.[PUBMED Abstract]
- Broaddus E, Topham A, Singh AD: Survival with retinoblastoma in the USA: 1975-2004. Br J Ophthalmol 93 (1): 24-7, 2009.[PUBMED Abstract]
- Shinohara ET, DeWees T, Perkins SM: Subsequent malignancies and their effect on survival in patients with retinoblastoma. Pediatr Blood Cancer 61 (1): 116-9, 2014.[PUBMED Abstract]
- Temming P, Arendt M, Viehmann A, et al.: Incidence of second cancers after radiotherapy and systemic chemotherapy in heritable retinoblastoma survivors: A report from the German reference center. Pediatr Blood Cancer 64 (1): 71-80, 2017.[PUBMED Abstract]
- Kleinerman RA, Tucker MA, Sigel BS, et al.: Patterns of Cause-Specific Mortality Among 2053 Survivors of Retinoblastoma, 1914-2016. J Natl Cancer Inst 111 (9): 961-969, 2019.[PUBMED Abstract]
- de Jong MC, Kors WA, de Graaf P, et al.: Trilateral retinoblastoma: a systematic review and meta-analysis. Lancet Oncol 15 (10): 1157-67, 2014.[PUBMED Abstract]
- Blach LE, McCormick B, Abramson DH, et al.: Trilateral retinoblastoma--incidence and outcome: a decade of experience. Int J Radiat Oncol Biol Phys 29 (4): 729-33, 1994.[PUBMED Abstract]
- Rodjan F, de Graaf P, Brisse HJ, et al.: Trilateral retinoblastoma: neuroimaging characteristics and value of routine brain screening on admission. J Neurooncol 109 (3): 535-44, 2012.[PUBMED Abstract]
- Kivelä T: Trilateral retinoblastoma: a meta-analysis of hereditary retinoblastoma associated with primary ectopic intracranial retinoblastoma. J Clin Oncol 17 (6): 1829-37, 1999.[PUBMED Abstract]
- Sirin S, de Jong MC, Galluzzi P, et al.: MRI-based assessment of the pineal gland in a large population of children aged 0-5 years and comparison with pineoblastoma: part II, the cystic gland. Neuroradiology 58 (7): 713-21, 2016.[PUBMED Abstract]
- Pham TT, Siebert E, Asbach P, et al.: Magnetic resonance imaging based morphologic evaluation of the pineal gland for suspected pineoblastoma in retinoblastoma patients and age-matched controls. J Neurol Sci 359 (1-2): 185-92, 2015.[PUBMED Abstract]
- MacCarthy A, Bayne AM, Brownbill PA, et al.: Second and subsequent tumours among 1927 retinoblastoma patients diagnosed in Britain 1951-2004. Br J Cancer 108 (12): 2455-63, 2013.[PUBMED Abstract]
- Dommering CJ, Marees T, van der Hout AH, et al.: RB1 mutations and second primary malignancies after hereditary retinoblastoma. Fam Cancer 11 (2): 225-33, 2012.[PUBMED Abstract]
- Fletcher O, Easton D, Anderson K, et al.: Lifetime risks of common cancers among retinoblastoma survivors. J Natl Cancer Inst 96 (5): 357-63, 2004.[PUBMED Abstract]
- Marees T, van Leeuwen FE, de Boer MR, et al.: Cancer mortality in long-term survivors of retinoblastoma. Eur J Cancer 45 (18): 3245-53, 2009.[PUBMED Abstract]
- Kleinerman RA, Yu CL, Little MP, et al.: Variation of second cancer risk by family history of retinoblastoma among long-term survivors. J Clin Oncol 30 (9): 950-7, 2012.[PUBMED Abstract]
- Wong FL, Boice JD, Abramson DH, et al.: Cancer incidence after retinoblastoma. Radiation dose and sarcoma risk. JAMA 278 (15): 1262-7, 1997.[PUBMED Abstract]
- Temming P, Arendt M, Viehmann A, et al.: How Eye-Preserving Therapy Affects Long-Term Overall Survival in Heritable Retinoblastoma Survivors. J Clin Oncol 34 (26): 3183-8, 2016.[PUBMED Abstract]
- Kleinerman RA, Tucker MA, Tarone RE, et al.: Risk of new cancers after radiotherapy in long-term survivors of retinoblastoma: an extended follow-up. J Clin Oncol 23 (10): 2272-9, 2005.[PUBMED Abstract]
- Sethi RV, Shih HA, Yeap BY, et al.: Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer 120 (1): 126-33, 2014.[PUBMED Abstract]
- Kleinerman RA, Tucker MA, Abramson DH, et al.: Risk of soft tissue sarcomas by individual subtype in survivors of hereditary retinoblastoma. J Natl Cancer Inst 99 (1): 24-31, 2007.[PUBMED Abstract]
- Abramson DH, Frank CM: Second nonocular tumors in survivors of bilateral retinoblastoma: a possible age effect on radiation-related risk. Ophthalmology 105 (4): 573-9; discussion 579-80, 1998.[PUBMED Abstract]
- Moll AC, Imhof SM, Schouten-Van Meeteren AY, et al.: Second primary tumors in hereditary retinoblastoma: a register-based study, 1945-1997: is there an age effect on radiation-related risk? Ophthalmology 108 (6): 1109-14, 2001.[PUBMED Abstract]
- Abramson DH, Melson MR, Dunkel IJ, et al.: Third (fourth and fifth) nonocular tumors in survivors of retinoblastoma. Ophthalmology 108 (10): 1868-76, 2001.[PUBMED Abstract]
- Marees T, van Leeuwen FE, Schaapveld M, et al.: Risk of third malignancies and death after a second malignancy in retinoblastoma survivors. Eur J Cancer 46 (11): 2052-8, 2010.[PUBMED Abstract]
- Turaka K, Shields CL, Meadows AT, et al.: Second malignant neoplasms following chemoreduction with carboplatin, etoposide, and vincristine in 245 patients with intraocular retinoblastoma. Pediatr Blood Cancer 59 (1): 121-5, 2012.[PUBMED Abstract]
- Gombos DS, Hungerford J, Abramson DH, et al.: Secondary acute myelogenous leukemia in patients with retinoblastoma: is chemotherapy a factor? Ophthalmology 114 (7): 1378-83, 2007.[PUBMED Abstract]
- Tamboli D, Topham A, Singh N, et al.: Retinoblastoma: A SEER Dataset Evaluation for Treatment Patterns, Survival, and Second Malignant Neoplasms. Am J Ophthalmol 160 (5): 953-8, 2015.[PUBMED Abstract]
- Rihani R, Bazzeh F, Faqih N, et al.: Secondary hematopoietic malignancies in survivors of childhood cancer: an analysis of 111 cases from the Surveillance, Epidemiology, and End Result-9 registry. Cancer 116 (18): 4385-94, 2010.[PUBMED Abstract]
- Yu CL, Tucker MA, Abramson DH, et al.: Cause-specific mortality in long-term survivors of retinoblastoma. J Natl Cancer Inst 101 (8): 581-91, 2009.[PUBMED Abstract]
- Aerts I, Pacquement H, Doz F, et al.: Outcome of second malignancies after retinoblastoma: a retrospective analysis of 25 patients treated at the Institut Curie. Eur J Cancer 40 (10): 1522-9, 2004.[PUBMED Abstract]
- Eng C, Li FP, Abramson DH, et al.: Mortality from second tumors among long-term survivors of retinoblastoma. J Natl Cancer Inst 85 (14): 1121-8, 1993.[PUBMED Abstract]
- Dunkel IJ, Gerald WL, Rosenfield NS, et al.: Outcome of patients with a history of bilateral retinoblastoma treated for a second malignancy: the Memorial Sloan-Kettering experience. Med Pediatr Oncol 30 (1): 59-62, 1998.[PUBMED Abstract]
- Marees T, Moll AC, Imhof SM, et al.: Risk of second malignancies in survivors of retinoblastoma: more than 40 years of follow-up. J Natl Cancer Inst 100 (24): 1771-9, 2008.[PUBMED Abstract]
- Moll AC, Imhof SM, Bouter LM, et al.: Second primary tumors in patients with retinoblastoma. A review of the literature. Ophthalmic Genet 18 (1): 27-34, 1997.[PUBMED Abstract]
- Friedman DN, Chou JF, Oeffinger KC, et al.: Chronic medical conditions in adult survivors of retinoblastoma: Results of the Retinoblastoma Survivor Study. Cancer 122 (5): 773-81, 2016.[PUBMED Abstract]
- Chojniak MM, Chojniak R, Testa ML, et al.: Abnormal orbital growth in children submitted to enucleation for retinoblastoma treatment. J Pediatr Hematol Oncol 34 (3): e102-5, 2012.[PUBMED Abstract]
- Oatts JT, Robbins JA, de Alba Campomanes AG: The effect of enucleation on orbital growth in patients with retinoblastoma. J AAPOS 21 (4): 309-312, 2017.[PUBMED Abstract]
- Abramson DH, Melson MR, Servodidio C: Visual fields in retinoblastoma survivors. Arch Ophthalmol 122 (9): 1324-30, 2004.[PUBMED Abstract]
- Demirci H, Shields CL, Meadows AT, et al.: Long-term visual outcome following chemoreduction for retinoblastoma. Arch Ophthalmol 123 (11): 1525-30, 2005.[PUBMED Abstract]
- Lambert MP, Shields C, Meadows AT: A retrospective review of hearing in children with retinoblastoma treated with carboplatin-based chemotherapy. Pediatr Blood Cancer 50 (2): 223-6, 2008.[PUBMED Abstract]
- Batra A, Thakar A, Bakhshi S: Ototoxicity in retinoblastoma survivors treated with carboplatin based chemotherapy: A cross-sectional study of 116 patients. Pediatr Blood Cancer 62 (11): 2060, 2015.[PUBMED Abstract]
- Qaddoumi I, Bass JK, Wu J, et al.: Carboplatin-associated ototoxicity in children with retinoblastoma. J Clin Oncol 30 (10): 1034-41, 2012.[PUBMED Abstract]
- Leahey A: A cautionary tale: dosing chemotherapy in infants with retinoblastoma. J Clin Oncol 30 (10): 1023-4, 2012.[PUBMED Abstract]
- Tumor Pathology of Retinoblastoma
-
Maturing cone precursor cells appear to be the cell of origin in human retinoblastoma.[ 1 ][ 2 ] Microscopically, the appearance of retinoblastoma depends on the degree of differentiation. Undifferentiated retinoblastoma is composed of small, round, densely packed cells with hypochromatic nuclei and scant cytoplasm. Several degrees of photoreceptor differentiation have been described and are characterized by distinctive arrangements of tumor cells, as follows:
Retinoblastomas are characterized by marked cell proliferation, as evidenced by high mitosis counts, extremely high MIB-1 labeling indices, and strong diffuse nuclear immunoreactivity for CRX, a useful marker to discriminate retinoblastoma from other malignant, small, round cell tumors.[ 3 ][ 4 ]
Cavitary retinoblastoma, a rare variant of retinoblastoma, has ophthalmoscopically visible lucent cavities within the tumor. The cavitary spaces appear hollow on ultrasonography and hypofluorescent on angiography. Histopathologically, the cavitary spaces have been shown to represent areas of photoreceptor differentiation.[ 5 ] These tumors have been associated with minimal visible tumor response to chemotherapy, which is thought to be a sign of tumor differentiation.[ 6 ]
A pathologist experienced in ocular pathology and retinoblastoma should examine the enucleated specimen, particularly to determine risk features of extraocular dissemination (refer to the Treatment of Intraocular Retinoblastoma section of this summary for more information).
参考文献- Xu XL, Singh HP, Wang L, et al.: Rb suppresses human cone-precursor-derived retinoblastoma tumours. Nature 514 (7522): 385-8, 2014.[PUBMED Abstract]
- Singh HP, Wang S, Stachelek K, et al.: Developmental stage-specific proliferation and retinoblastoma genesis in RB-deficient human but not mouse cone precursors. Proc Natl Acad Sci U S A 115 (40): E9391-E9400, 2018.[PUBMED Abstract]
- Terry J, Calicchio ML, Rodriguez-Galindo C, et al.: Immunohistochemical expression of CRX in extracranial malignant small round cell tumors. Am J Surg Pathol 36 (8): 1165-9, 2012.[PUBMED Abstract]
- Schwimer CJ, Prayson RA: Clinicopathologic study of retinoblastoma including MIB-1, p53, and CD99 immunohistochemistry. Ann Diagn Pathol 5 (3): 148-54, 2001.[PUBMED Abstract]
- Palamar M, Pirondini C, Shields CL, et al.: Cavitary retinoblastoma: ultrasonographic and fluorescein angiographic findings in 3 cases. Arch Ophthalmol 126 (11): 1598-600, 2008.[PUBMED Abstract]
- Mashayekhi A, Shields CL, Eagle RC, et al.: Cavitary changes in retinoblastoma: relationship to chemoresistance. Ophthalmology 112 (6): 1145-50, 2005.[PUBMED Abstract]
- Staging and Grouping Systems for Retinoblastoma
-
The staging of patients with retinoblastoma requires close coordination of radiologists, pediatric oncologists, and ophthalmologists. Several staging and grouping systems have been proposed for retinoblastoma.[ 1 ] Overall assessment of retinoblastoma extension is documented by staging systems; the extent of intraocular disease, which is relevant for ocular salvage, is documented by grouping systems. For treatment purposes, retinoblastoma is categorized into intraocular and extraocular disease.
Intraocular Retinoblastoma
Intraocular retinoblastoma is localized to the eye; it may be confined to the retina or may extend to involve other structures such as the choroid, ciliary body, anterior chamber, and optic nerve head. Intraocular retinoblastoma, however, does not extend beyond the eye into the tissues around the eye or to other parts of the body.
Extraocular Retinoblastoma
Extraocular retinoblastoma extends beyond the eye. It may be confined to the tissues around the eye (orbital retinoblastoma), it may have spread to the central nervous system, or it may have spread systemically to the bone marrow or lymph nodes (metastatic retinoblastoma).
Staging Systems
American Joint Committee on Cancer (AJCC) staging system
Several staging systems have been proposed over the years. The newest standard for state-mandated cancer reporting to the North American Association of Cancer Registries requires AJCC staging, according to the 8th edition of the staging manual. This affects cases diagnosed in 2018 and thereafter. Retinoblastoma staging is the first to acknowledge the role of genetic predisposition by incorporating an H category. H1 refers to patients with bilateral or trilateral retinoblastoma, a family history of retinoblastoma, or the presence of an RB1 mutation.[ 2 ]
International Retinoblastoma Staging System (IRSS)
The more simplified IRSS has been proposed by an international consortium of ophthalmologists and pediatric oncologists;[ 3 ] it is more widely used in the clinical setting than the AJCC staging system (refer to Table 2). A retrospective German study found that the IRSS predicted survival in 633 children with heritable retinoblastoma, 582 of whom presented with IRSS stage 0 or I disease.[ 4 ]
Table 2. International Retinoblastoma Staging System Stage Description CNS = central nervous system; CSF = cerebrospinal fluid. 0 Eye has not been enucleated and no dissemination of disease (refer to the International Classification of Retinoblastoma section of this summary for more information). I Eye enucleated, completely resected histologically II Eye enucleated, microscopic residual tumor III Regional extension a. Overt orbital disease b. Preauricular or cervical lymph node extension IV Metastatic disease a. Hematogenous metastasis (without CNS involvement) —Single lesion —Multiple lesions b. CNS extension (with or without any other site of regional or metastatic disease) —Prechiasmatic lesion —CNS mass —Leptomeningeal and CSF disease Grouping Systems
The following grouping systems are relevant for assessment of intraocular disease extension and are helpful predictors of ocular salvage:
International Classification of Retinoblastoma
The International Classification of Retinoblastoma grouping system was developed with the goal of providing a simpler, more user-friendly classification that is more applicable to current therapies. This newer system is based on the extent of tumor seeding within the vitreous cavity and subretinal space, rather than on tumor size and location (refer to Table 3). This system seems to be a better predictor of treatment success.[ 5 ][ 6 ][ 7 ][ 8 ] The International Classification of Retinoblastoma system may also help predict high-risk histopathology. In a study of more than 500 patients with retinoblastoma, histopathologic evidence of high-risk disease was noted in 17% of Group D eyes and 24% of Group E eyes. This can be helpful in counseling parents regarding the potential need for postoperative systemic therapy.[ 9 ]
Table 3. The International Classification of Retinoblastoma Grouping System Group Definition Group A Small intraretinal tumors away from the foveola and disc. All tumors are 3 mm or smaller in greatest dimension, confined to the retina and All tumors are located further than 3 mm from the foveola and 1.5 mm from the optic disc. Group B All remaining discrete tumors confined to the retina. All other tumors confined to the retina not in Group A. Tumor-associated subretinal fluid less than 3 mm from the tumor with no subretinal seeding. Tumor located closer than 3 mm to the optic nerve or fovea. Group C Discrete local disease with minimal subretinal or vitreous seeding. Tumor(s) are discrete. Subretinal fluid, present or past, without seeding involving up to one-fourth of the retina. Local fine vitreous seeding may be present close to the discrete tumor. Local subretinal seeding less than 3 mm (2 DD) from the tumor. Group D Diffuse disease with significant vitreous or subretinal seeding. Tumor(s) may be massive or diffuse. Subretinal fluid present or past without seeding, involving up to total retinal detachment. Diffuse or massive vitreous disease may include greasy seeds or avascular tumor masses. Diffuse subretinal seeding may include subretinal plaques or tumor nodules. Group E Presence of any one or more of the following poor prognosis features: Tumor touching the lens. Tumor anterior to anterior vitreous face involving ciliary body or anterior segment. Diffuse infiltrating retinoblastoma. Neovascular glaucoma. Opaque media from hemorrhage. Tumor necrosis with aseptic orbital cellulites. Phthisis bulbi. Reese-Ellsworth Classification for Intraocular Tumors
Reese and Ellsworth developed a classification system for intraocular retinoblastoma that has been shown to have prognostic significance for maintenance of sight and control of local disease at a time when surgery and external-beam radiation therapy were the primary treatment options. However, developments in the conservative management of intraocular retinoblastoma have made the Reese-Ellsworth grouping system less predictive for eye salvage and less helpful in guiding treatment.[ 7 ] This grouping system is seldom used and serves largely as a historical reference.
参考文献- Chantada GL, Sampor C, Bosaleh A, et al.: Comparison of staging systems for extraocular retinoblastoma: analysis of 533 patients. JAMA Ophthalmol 131 (9): 1127-34, 2013.[PUBMED Abstract]
- Mallipatna A, Gallie BL, Chevez-Barrios P, et al.: Retinoblastoma. In: Amin MB, Edge SB, Greene FL, et al., eds.: AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017, pp. 819-31.[PUBMED Abstract]
- Chantada G, Doz F, Antoneli CB, et al.: A proposal for an international retinoblastoma staging system. Pediatr Blood Cancer 47 (6): 801-5, 2006.[PUBMED Abstract]
- Temming P, Arendt M, Viehmann A, et al.: How Eye-Preserving Therapy Affects Long-Term Overall Survival in Heritable Retinoblastoma Survivors. J Clin Oncol 34 (26): 3183-8, 2016.[PUBMED Abstract]
- Murphree L: Staging and grouping of retinoblastoma. In: Singh A, Damato B: Clinical Ophthalmic Oncology. Philadelphia, Pa: Saunders Elsevier, 2007, pp 422-7.[PUBMED Abstract]
- Zage PE, Reitman AJ, Seshadri R, et al.: Outcomes of a two-drug chemotherapy regimen for intraocular retinoblastoma. Pediatr Blood Cancer 50 (3): 567-72, 2008.[PUBMED Abstract]
- Shields CL, Mashayekhi A, Au AK, et al.: The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology 113 (12): 2276-80, 2006.[PUBMED Abstract]
- Novetsky DE, Abramson DH, Kim JW, et al.: Published international classification of retinoblastoma (ICRB) definitions contain inconsistencies--an analysis of impact. Ophthalmic Genet 30 (1): 40-4, 2009.[PUBMED Abstract]
- Kaliki S, Shields CL, Rojanaporn D, et al.: High-risk retinoblastoma based on international classification of retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology 120 (5): 997-1003, 2013.[PUBMED Abstract]
- Treatment Option Overview for Retinoblastoma
-
Treatment planning by a multidisciplinary team of cancer specialists—including a pediatric oncologist, ophthalmologist, and radiation oncologist—with experience treating ocular tumors of childhood is required to optimize treatment outcomes.[ 1 ] Evaluation at specialized centers is highly recommended before the initiation of treatment to improve the likelihood of ocular salvage and vision preservation.
The goals of therapy include the following:
Many treatments considered to be standard of care have not been studied in a randomized fashion.
Treatment of retinoblastoma depends on the intraocular and extraocular disease burden, disease laterality, germline RB1 gene status, and the potential for preserving vision. For patients presenting with intraocular disease, particularly those with bilateral eye involvement, a conservative approach consisting of tumor reduction with intravenous or ophthalmic artery chemotherapy, coupled with aggressive local therapy, may result in high ocular salvage rates.[ 2 ] Radiation therapy, one of the most effective treatments in retinoblastoma, is usually reserved for cases of intraocular or extraocular disease progression.
A risk-adapted, judicious combination of the following therapeutic options should be considered:
The treatment options for intraocular, extraocular, and recurrent retinoblastoma are described in Table 4.
Enucleation
Upfront removal of the eye is indicated for large tumors filling the vitreous for which there is little or no likelihood of restoring vision, in cases of extension to the anterior chamber, or in the presence of neovascular glaucoma. Patients must be monitored closely for orbital recurrence of disease, particularly in the first 2 years after enucleation.[ 3 ][Level of evidence: 3iiA] Enucleation is also used as a salvage treatment in cases of disease progression or recurrence in patients receiving eye-salvage management. The pathology specimen must be carefully examined to identify patients who are at high risk of extraocular dissemination and who may require adjuvant chemotherapy.
Enucleation in patients younger than 3 years does not allow for the proper enlargement of the orbit during subsequent development, causing asymmetry of the final orbital size.[ 4 ]
Local Treatment (Cryotherapy, Laser Therapy, and Brachytherapy)
For patients undergoing eye-salvage treatment, aggressive local therapy is always required. Local treatment is administered by the ophthalmologist directly to the tumor.
Systemic Chemotherapy
Systemic chemotherapy plays a role in the following:
Ophthalmic Artery Infusion of Chemotherapy (Intra-arterial Chemotherapy)
Direct delivery of chemotherapy into the eye via cannulation of the ophthalmic artery is a feasible and effective method for ocular salvage.
Melphalan is the most common and most effective agent used for intra-arterial chemotherapy. It is often combined with topotecan or carboplatin when responses are suboptimal or there is very advanced intraocular disease.[ 19 ][ 20 ]
For patients with treatment-naive eyes, the 2-year radiation-free ocular survival rate is 86% to 90%.[ 19 ][ 20 ] Outcome after intra-arterial chemotherapy correlates with the extent of intraocular burden, as follows:
Patients with bilateral disease can undergo tandem intra-arterial chemotherapy administration.[ 24 ] In those circumstances, patients are at higher risk of systemic toxicity caused by melphalan exposure,[ 25 ] and single-agent carboplatin may be used to treat the less-advanced eye during the tandem procedure.[ 26 ] For neonates and very young infants in whom the cannulization of the ophthalmic artery is not feasible, bridge treatment with single-agent systemic carboplatin until the infant is aged 3 months or weighs 6 kg, followed by consolidation with intra-arterial chemotherapy, has been shown to be very effective, with a 1-year radiation-free ocular survival rate of 95%.[ 27 ]
The addition of intravitreal chemotherapy to intra-arterial chemotherapy appears to markedly improve the overall effectiveness in eyes with vitreous seeds, especially those with vitreous seed clouds (refer to the Intravitreal Chemotherapy section of this summary for more information).[ 19 ][ 28 ][ 29 ] In patients presenting with total retinal detachment, ophthalmic artery chemosurgery has been shown to promote retinal reattachment.[ 30 ]
Complications related to intra-arterial chemotherapy include the following:[ 20 ]
Major vascular complications related to the procedure are very rare; no strokes or significant acute neurological events have been reported by the most experienced groups.[ 19 ][ 20 ][ 32 ] However, stenosis of the ophthalmic artery and occlusion of the retinal artery have been documented;[ 32 ] the risk of thrombosis is significantly increased in children with thrombophilia.[ 33 ]
The impact of the intraocular vascular changes on vision has not been fully assessed because of the young age of the first cohorts of patients treated. Most patients do not have substantial electroretinographic changes,[ 34 ] and preservation of central vision has been reported.[ 35 ] A proportion of patients with abnormal electroretinograms (ERGs) with or without retinal detachment may have improved ERGs in the years after intra-arterial chemotherapy.[ 36 ] However, in patients with heavily pretreated eyes, intensive intra-arterial chemotherapy may result in worsening of retinal function.[ 23 ]
Another risk associated with intra-arterial chemotherapy is the exposure to ionizing radiation during fluoroscopy. Mean total radiation doses of 42.3 mGy have been reported in very experienced centers.[ 37 ] After multiple procedures, cumulative doses can reach 0.1 to 0.2 Gy, which can be cataractogenic and potentially carcinogenic in this susceptible population.[ 38 ] There is no increase in the incidence of second malignancies;[ 39 ][ 40 ] however, longer follow-up will be required to fully ascertain the risks associated with the procedure.
The risk of metastatic progression with direct ocular delivery of chemotherapy appears to be very low;[ 2 ] however, up to 20 cases of patients treated with intra-arterial chemotherapy who subsequently developed metastases have been reported.[ 20 ]
Intravitreal Chemotherapy
Studies suggest that direct intravitreal injection of melphalan or topotecan may be effective in controlling active vitreous seeds.[ 41 ][ 42 ]; [ 43 ][Level of evidence: 3iiDi]; [ 44 ][Level of evidence: 3iiiDiii] A retrospective study of 264 eyes (250 children) treated with intravitreal melphalan for vitreous seeds over a two-decade period reported a 68% complete remission rate. There was a low incidence of extraocular spread as a result of the injection that occurred in children with high-risk features.[ 45 ][Level of evidence: 3iiD]
Because of initial concerns about the potential for tumor dissemination, the use of intravitreal chemotherapy was limited. However, additional reports have estimated that the proportion of patients with extraocular tumor spread, as the result of intravitreal injection, is negligible.[ 46 ][ 47 ] While this procedure is safe and well tolerated, recent studies have shown a direct correlation between the number of injections and a decrease in retinal function, as measured by ERG.[ 47 ]; [ 48 ][Level of evidence: 3iiiDiv]
Preliminary data seem to support that intra-arterial chemotherapy plus intravitreal chemotherapy (as needed for vitreous seeding) may improve globe salvage in eyes with advanced retinoblastoma when compared with children who were treated in earlier years with intra-arterial chemotherapy alone.[ 47 ]; [ 28 ][Level of evidence: 3iiDii] Compared with the children treated in the earlier era, children treated in the later era received a combination of intra-arterial and intra-vitreal chemotherapy, which demonstrated shorter time to regression, fewer recurrences, fewer enucleations, and no increased toxicity, including no difference in loss of retinal function as measured by ERG.[ 29 ][Level of evidence: 3iiDiv]
As experience with the use of intra-vitreal chemotherapy expands, studies have demonstrated its efficacy in controlling subretinal seeds and recurrent retinal tumors, suggesting a potential role beyond the control of vitreous seeds as an adjunctive therapy in the globe-sparing treatment of retinoblastoma.[ 49 ]
Subtenon Chemotherapy (Subconjunctival Chemotherapy)
Periocular delivery of carboplatin results in high intraocular concentrations of the agent, and this treatment is often used in ocular salvage approaches, particularly when there is a high vitreal tumor burden. Carboplatin is administered by the treating ophthalmologist into the subtenon space, and it is generally used in conjunction with systemic chemotherapy and local ophthalmic therapies for patients with vitreous disease.[ 50 ] Responses have also been noted with subtenon topotecan.[ 29 ][ 51 ]
With the development of new treatments for retinoblastoma, such as intra-arterial and intravitreal delivery of chemotherapy, subtenon chemotherapy is being used less often in the clinical setting.
Radiation Therapy
Special Considerations for the Treatment of Children with Cancer
Cancer in children and adolescents is rare, although the overall incidence of childhood cancer has been slowly increasing since 1975.[ 60 ] Children and adolescents with cancer should be referred to medical centers that have a multidisciplinary team of cancer specialists with experience treating the cancers that occur during childhood and adolescence. This multidisciplinary team approach is particularly important in the management of retinoblastoma; this approach incorporates the skills of the following health care professionals and others to ensure that children receive treatment, supportive care, and rehabilitation that will provide optimal survival and quality of life:
Guidelines for pediatric cancer centers and their role in the treatment of pediatric patients with cancer have been outlined by the American Academy of Pediatrics.[ 61 ] At these pediatric cancer centers, clinical trials are available for most types of cancer that occur in children and adolescents, and the opportunity to participate in these trials is offered to most patients and their families. Clinical trials for children and adolescents with cancer are generally designed to compare potentially better therapy with therapy that is currently accepted as standard. Most of the progress made in identifying curative therapies for childhood cancers has been achieved through clinical trials. Information about ongoing clinical trials is available from the NCI website.
Dramatic improvements in survival have been achieved for children and adolescents with cancer.[ 60 ][ 62 ][ 63 ] Between 1975 and 2010, childhood cancer mortality decreased by more than 50%.[ 60 ][ 62 ][ 63 ] Childhood and adolescent cancer survivors require close monitoring because cancer therapy side effects may persist or develop months or years after treatment. (Refer to the PDQ summary on Late Effects of Treatment for Childhood Cancer for specific information about the incidence, type, and monitoring of late effects in childhood and adolescent cancer survivors.)
参考文献- Chintagumpala M, Chevez-Barrios P, Paysse EA, et al.: Retinoblastoma: review of current management. Oncologist 12 (10): 1237-46, 2007.[PUBMED Abstract]
- Abramson DH, Fabius AW, Issa R, et al.: Advanced Unilateral Retinoblastoma: The Impact of Ophthalmic Artery Chemosurgery on Enucleation Rate and Patient Survival at MSKCC. PLoS One 10 (12): e0145436, 2015.[PUBMED Abstract]
- Kim JW, Kathpalia V, Dunkel IJ, et al.: Orbital recurrence of retinoblastoma following enucleation. Br J Ophthalmol 93 (4): 463-7, 2009.[PUBMED Abstract]
- Oatts JT, Robbins JA, de Alba Campomanes AG: The effect of enucleation on orbital growth in patients with retinoblastoma. J AAPOS 21 (4): 309-312, 2017.[PUBMED Abstract]
- Shields CL, Santos MC, Diniz W, et al.: Thermotherapy for retinoblastoma. Arch Ophthalmol 117 (7): 885-93, 1999.[PUBMED Abstract]
- Francis JH, Abramson DH, Brodie SE, et al.: Indocyanine green enhanced transpupillary thermotherapy in combination with ophthalmic artery chemosurgery for retinoblastoma. Br J Ophthalmol 97 (2): 164-8, 2013.[PUBMED Abstract]
- Chantada GL, Fandiño AC, Guitter MR, et al.: Results of a prospective study for the treatment of unilateral retinoblastoma. Pediatr Blood Cancer 55 (1): 60-6, 2010.[PUBMED Abstract]
- Aerts I, Sastre-Garau X, Savignoni A, et al.: Results of a multicenter prospective study on the postoperative treatment of unilateral retinoblastoma after primary enucleation. J Clin Oncol 31 (11): 1458-63, 2013.[PUBMED Abstract]
- Kaliki S, Shields CL, Rojanaporn D, et al.: High-risk retinoblastoma based on international classification of retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology 120 (5): 997-1003, 2013.[PUBMED Abstract]
- Sullivan EM, Wilson MW, Billups CA, et al.: Pathologic risk-based adjuvant chemotherapy for unilateral retinoblastoma following enucleation. J Pediatr Hematol Oncol 36 (6): e335-40, 2014.[PUBMED Abstract]
- Dunkel IJ, Khakoo Y, Kernan NA, et al.: Intensive multimodality therapy for patients with stage 4a metastatic retinoblastoma. Pediatr Blood Cancer 55 (1): 55-9, 2010.[PUBMED Abstract]
- Matsubara H, Makimoto A, Higa T, et al.: A multidisciplinary treatment strategy that includes high-dose chemotherapy for metastatic retinoblastoma without CNS involvement. Bone Marrow Transplant 35 (8): 763-6, 2005.[PUBMED Abstract]
- Rodriguez-Galindo C, Wilson MW, Haik BG, et al.: Treatment of metastatic retinoblastoma. Ophthalmology 110 (6): 1237-40, 2003.[PUBMED Abstract]
- Kremens B, Wieland R, Reinhard H, et al.: High-dose chemotherapy with autologous stem cell rescue in children with retinoblastoma. Bone Marrow Transplant 31 (4): 281-4, 2003.[PUBMED Abstract]
- Rodriguez-Galindo C, Orbach DB, VanderVeen D: Retinoblastoma. Pediatr Clin North Am 62 (1): 201-23, 2015.[PUBMED Abstract]
- Zhao J, Dimaras H, Massey C, et al.: Pre-enucleation chemotherapy for eyes severely affected by retinoblastoma masks risk of tumor extension and increases death from metastasis. J Clin Oncol 29 (7): 845-51, 2011.[PUBMED Abstract]
- Berry JL, Kogachi K, Aziz HA, et al.: Risk of metastasis and orbital recurrence in advanced retinoblastoma eyes treated with systemic chemoreduction versus primary enucleation. Pediatr Blood Cancer 64 (4): , 2017.[PUBMED Abstract]
- Wilson MW, Haik BG, Billups CA, et al.: Incidence of new tumor formation in patients with hereditary retinoblastoma treated with primary systemic chemotherapy: is there a preventive effect? Ophthalmology 114 (11): 2077-82, 2007.[PUBMED Abstract]
- Francis JH, Levin AM, Zabor EC, et al.: Ten-year experience with ophthalmic artery chemosurgery: Ocular and recurrence-free survival. PLoS One 13 (5): e0197081, 2018.[PUBMED Abstract]
- Yousef YA, Soliman SE, Astudillo PPP, et al.: Intra-arterial Chemotherapy for Retinoblastoma: A Systematic Review. JAMA Ophthalmol 134 (5): 584-591, 2016.[PUBMED Abstract]
- Abramson DH, Daniels AB, Marr BP, et al.: Intra-Arterial Chemotherapy (Ophthalmic Artery Chemosurgery) for Group D Retinoblastoma. PLoS One 11 (1): e0146582, 2016.[PUBMED Abstract]
- Shields CL, Kaliki S, Al-Dahmash S, et al.: Management of advanced retinoblastoma with intravenous chemotherapy then intra-arterial chemotherapy as alternative to enucleation. Retina 33 (10): 2103-9, 2013 Nov-Dec.[PUBMED Abstract]
- Marr BP, Brodie SE, Dunkel IJ, et al.: Three-drug intra-arterial chemotherapy using simultaneous carboplatin, topotecan and melphalan for intraocular retinoblastoma: preliminary results. Br J Ophthalmol 96 (10): 1300-3, 2012.[PUBMED Abstract]
- Abramson DH, Marr BP, Francis JH, et al.: Simultaneous Bilateral Ophthalmic Artery Chemosurgery for Bilateral Retinoblastoma (Tandem Therapy). PLoS One 11 (6): e0156806, 2016.[PUBMED Abstract]
- Schaiquevich P, Buitrago E, Taich P, et al.: Pharmacokinetic analysis of melphalan after superselective ophthalmic artery infusion in preclinical models and retinoblastoma patients. Invest Ophthalmol Vis Sci 53 (7): 4205-12, 2012.[PUBMED Abstract]
- Francis JH, Gobin YP, Brodie SE, et al.: Experience of intra-arterial chemosurgery with single agent carboplatin for retinoblastoma. Br J Ophthalmol 96 (9): 1270-1, 2012.[PUBMED Abstract]
- Gobin YP, Dunkel IJ, Marr BP, et al.: Combined, sequential intravenous and intra-arterial chemotherapy (bridge chemotherapy) for young infants with retinoblastoma. PLoS One 7 (9): e44322, 2012.[PUBMED Abstract]
- Shields CL, Alset AE, Say EA, et al.: Retinoblastoma Control With Primary Intra-arterial Chemotherapy: Outcomes Before and During the Intravitreal Chemotherapy Era. J Pediatr Ophthalmol Strabismus 53 (5): 275-84, 2016.[PUBMED Abstract]
- Francis JH, Iyer S, Gobin YP, et al.: Retinoblastoma Vitreous Seed Clouds (Class 3): A Comparison of Treatment with Ophthalmic Artery Chemosurgery with or without Intravitreous and Periocular Chemotherapy. Ophthalmology 124 (10): 1548-1555, 2017.[PUBMED Abstract]
- Rowlands MA, Mondesire-Crump I, Levin A, et al.: Total retinal detachments due to retinoblastoma: Outcomes following intra-arterial chemotherapy/ophthalmic artery chemosurgery. PLoS One 13 (4): e0195395, 2018.[PUBMED Abstract]
- Shields CL, Say EAT, Pefkianaki M, et al.: RHEGMATOGENOUS RETINAL DETACHMENT AFTER INTRAARTERIAL CHEMOTHERAPY FOR RETINOBLASTOMA: The 2016 Founders Award Lecture. Retina 37 (8): 1441-1450, 2017.[PUBMED Abstract]
- Shields CL, Bianciotto CG, Jabbour P, et al.: Intra-arterial chemotherapy for retinoblastoma: report No. 2, treatment complications. Arch Ophthalmol 129 (11): 1407-15, 2011.[PUBMED Abstract]
- Francis JH, Gobin YP, Nagiel A, et al.: Thrombophilia in patients with retinoblastoma receiving ophthalmic artery chemosurgery. Arch Ophthalmol 130 (12): 1605-8, 2012.[PUBMED Abstract]
- Abramson DH: Chemosurgery for retinoblastoma: what we know after 5 years. Arch Ophthalmol 129 (11): 1492-4, 2011.[PUBMED Abstract]
- Brodie SE, Munier FL, Francis JH, et al.: Persistence of retinal function after intravitreal melphalan injection for retinoblastoma. Doc Ophthalmol 126 (1): 79-84, 2013.[PUBMED Abstract]
- Abdelhakim AH, Francis JH, Marr BP, et al.: Retinal reattachment and ERG recovery after ophthalmic artery chemosurgery for advanced retinoblastoma in eyes with minimal baseline retinal function. Br J Ophthalmol 101 (5): 623-628, 2017.[PUBMED Abstract]
- Boddu SR, Abramson DH, Marr BP, et al.: Selective ophthalmic artery chemosurgery (SOAC) for retinoblastoma: fluoroscopic time and radiation dose parameters. A baseline study. J Neurointerv Surg 9 (11): 1107-1112, 2017.[PUBMED Abstract]
- Vijayakrishnan R, Shields CL, Ramasubramanian A, et al.: Irradiation toxic effects during intra-arterial chemotherapy for retinoblastoma: should we be concerned? Arch Ophthalmol 128 (11): 1427-31, 2010.[PUBMED Abstract]
- Suzuki S, Yamane T, Mohri M, et al.: Selective ophthalmic arterial injection therapy for intraocular retinoblastoma: the long-term prognosis. Ophthalmology 118 (10): 2081-7, 2011.[PUBMED Abstract]
- Habib LA, Francis JH, Fabius AW, et al.: Second primary malignancies in retinoblastoma patients treated with intra-arterial chemotherapy: the first 10 years. Br J Ophthalmol 102 (2): 272-275, 2018.[PUBMED Abstract]
- Francis JH, Abramson DH, Gaillard MC, et al.: The classification of vitreous seeds in retinoblastoma and response to intravitreal melphalan. Ophthalmology 122 (6): 1173-9, 2015.[PUBMED Abstract]
- Shields CL, Manjandavida FP, Arepalli S, et al.: Intravitreal melphalan for persistent or recurrent retinoblastoma vitreous seeds: preliminary results. JAMA Ophthalmol 132 (3): 319-25, 2014.[PUBMED Abstract]
- Ghassemi F, Shields CL: Intravitreal melphalan for refractory or recurrent vitreous seeding from retinoblastoma. Arch Ophthalmol 130 (10): 1268-71, 2012.[PUBMED Abstract]
- Munier FL, Gaillard MC, Balmer A, et al.: Intravitreal chemotherapy for vitreous disease in retinoblastoma revisited: from prohibition to conditional indications. Br J Ophthalmol 96 (8): 1078-83, 2012.[PUBMED Abstract]
- Suzuki S, Aihara Y, Fujiwara M, et al.: Intravitreal injection of melphalan for intraocular retinoblastoma. Jpn J Ophthalmol 59 (3): 164-72, 2015.[PUBMED Abstract]
- Smith SJ, Smith BD: Evaluating the risk of extraocular tumour spread following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol 97 (10): 1231-6, 2013.[PUBMED Abstract]
- Francis JH, Brodie SE, Marr B, et al.: Efficacy and Toxicity of Intravitreous Chemotherapy for Retinoblastoma: Four-Year Experience. Ophthalmology 124 (4): 488-495, 2017.[PUBMED Abstract]
- Smith SJ, Smith BD, Mohney BG: Ocular side effects following intravitreal injection therapy for retinoblastoma: a systematic review. Br J Ophthalmol 98 (3): 292-7, 2014.[PUBMED Abstract]
- Abramson DH, Ji X, Francis JH, et al.: Intravitreal chemotherapy in retinoblastoma: expanded use beyond intravitreal seeds. Br J Ophthalmol 103 (4): 488-493, 2019.[PUBMED Abstract]
- Marr BP, Dunkel IJ, Linker A, et al.: Periocular carboplatin for retinoblastoma: long-term report (12 years) on efficacy and toxicity. Br J Ophthalmol 96 (6): 881-3, 2012.[PUBMED Abstract]
- Mallipatna AC, Dimaras H, Chan HS, et al.: Periocular topotecan for intraocular retinoblastoma. Arch Ophthalmol 129 (6): 738-45, 2011.[PUBMED Abstract]
- Krasin MJ, Crawford BT, Zhu Y, et al.: Intensity-modulated radiation therapy for children with intraocular retinoblastoma: potential sparing of the bony orbit. Clin Oncol (R Coll Radiol) 16 (3): 215-22, 2004.[PUBMED Abstract]
- Reisner ML, Viégas CM, Grazziotin RZ, et al.: Retinoblastoma--comparative analysis of external radiotherapy techniques, including an IMRT technique. Int J Radiat Oncol Biol Phys 67 (3): 933-41, 2007.[PUBMED Abstract]
- Lee CT, Bilton SD, Famiglietti RM, et al.: Treatment planning with protons for pediatric retinoblastoma, medulloblastoma, and pelvic sarcoma: how do protons compare with other conformal techniques? Int J Radiat Oncol Biol Phys 63 (2): 362-72, 2005.[PUBMED Abstract]
- Mouw KW, Sethi RV, Yeap BY, et al.: Proton radiation therapy for the treatment of retinoblastoma. Int J Radiat Oncol Biol Phys 90 (4): 863-9, 2014.[PUBMED Abstract]
- Sethi RV, Shih HA, Yeap BY, et al.: Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer 120 (1): 126-33, 2014.[PUBMED Abstract]
- Shields CL, Shields JA, Cater J, et al.: Plaque radiotherapy for retinoblastoma: long-term tumor control and treatment complications in 208 tumors. Ophthalmology 108 (11): 2116-21, 2001.[PUBMED Abstract]
- Merchant TE, Gould CJ, Wilson MW, et al.: Episcleral plaque brachytherapy for retinoblastoma. Pediatr Blood Cancer 43 (2): 134-9, 2004.[PUBMED Abstract]
- Shields CL, Mashayekhi A, Sun H, et al.: Iodine 125 plaque radiotherapy as salvage treatment for retinoblastoma recurrence after chemoreduction in 84 tumors. Ophthalmology 113 (11): 2087-92, 2006.[PUBMED Abstract]
- Smith MA, Altekruse SF, Adamson PC, et al.: Declining childhood and adolescent cancer mortality. Cancer 120 (16): 2497-506, 2014.[PUBMED Abstract]
- Corrigan JJ, Feig SA; American Academy of Pediatrics: Guidelines for pediatric cancer centers. Pediatrics 113 (6): 1833-5, 2004.[PUBMED Abstract]
- Childhood cancer. In: Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2010. Bethesda, Md: National Cancer Institute, 2013, Section 28. Also available online. Last accessed August 09, 2019.[PUBMED Abstract]
- Childhood cancer by the ICCC. In: Howlader N, Noone AM, Krapcho M, et al., eds.: SEER Cancer Statistics Review, 1975-2010. Bethesda, Md: National Cancer Institute, 2013, Section 29. Also available online. Last accessed August 09, 2019.[PUBMED Abstract]
- Treatment of Intraocular Retinoblastoma
-
Treatment of Unilateral Intraocular Retinoblastoma
Treatment options for unilateral intraocular retinoblastoma include the following:
- Enucleation for large intraocular tumors, with or without adjuvant chemotherapy based on pathology risk, when the eye cannot be saved.
- Conservative ocular salvage approaches when the eye and vision can be saved:
Enucleation with or without adjuvant chemotherapy
Because unilateral disease is usually massive and often there is no expectation that useful vision can be preserved, up-front surgery (enucleation) is commonly performed. Careful examination of the enucleated specimen by an experienced pathologist is necessary to determine whether high-risk features for metastatic disease are present. These high-risk features include the following:[ 1 ][ 2 ][ 3 ][ 4 ][ 5 ]
Pre-enucleation magnetic resonance imaging has low sensitivity and specificity for the detection of high-risk pathology.[ 6 ]
High-risk pathology has been associated with the presence of minimal dissemination in bone marrow and cerebrospinal fluid using quantitative polymerase chain reaction for detection of CRX or GD2 synthase. In a group of 96 children with nonmetastatic retinoblastoma and high-risk pathology, the 3-year disease-free survival was 78% for patients with detectable minimal dissemination, compared with 98% for those without detectable disease (P = .004).[ 7 ]
Systemic adjuvant therapy with vincristine, doxorubicin, and cyclophosphamide or with vincristine, carboplatin, and etoposide has been used to prevent the development of metastatic disease in patients with certain high-risk features assessed by pathologic review after enucleation.[ 3 ][ 8 ][ 9 ]; [ 10 ][Level of evidence: 2A]
Conservative ocular salvage approaches
Conservative ocular salvage approaches, such as systemic chemotherapy and local-control treatments, may be offered in an attempt to save the eye and preserve vision.[ 11 ] Ocular salvage rates correlate with intraocular grouping. While the possibility of saving the eye without the use of external-beam radiation therapy (EBRT) exceeds 80% for children with early intraocular disease, the ocular outcomes for children with advanced intraocular disease are poor, with less than 40% ocular salvage rates, even after the use of EBRT.[ 12 ]
Thus, caution must be exerted with extended systemic chemotherapy administration and delayed enucleation when tumor control does not appear to be possible, particularly for Group E eyes. Pre-enucleation chemotherapy for eyes with advanced intraocular disease may result in downstaging and underestimate the pathological evidence of extraretinal and extraocular disease, thus increasing the risk of dissemination.[ 13 ]
The delivery of chemotherapy via ophthalmic artery cannulation as initial treatment for advanced unilateral retinoblastoma appears to be more effective than does systemic chemotherapy for chemoreduction, particularly for Group D eyes.[ 14 ][ 15 ]; [ 16 ][Level of evidence: 3iiDiv] In the setting of a multidisciplinary state-of-the-art center, intra-arterial chemotherapy may result in ocular salvage rates of approximately 80% to 90% for patients with advanced intraocular unilateral retinoblastoma.[ 15 ][ 16 ][ 17 ][ 18 ] (Refer to the Ophthalmic Artery Infusion of Chemotherapy [Intra-arterial Chemotherapy] section of this summary for more information.)
Because a proportion of children who present with unilateral retinoblastoma will eventually develop disease in the opposite eye, these children undergo genetic counseling and testing and periodic examinations of the unaffected eye, regardless of the treatment they receive. Asynchronous bilateral disease occurs most frequently in patients with affected parents and in children diagnosed during the first months of life.
Treatment of Bilateral Intraocular Retinoblastoma
The goal of therapy for bilateral retinoblastoma is ocular and vision preservation and the delay or avoidance of EBRT and enucleation.
Treatment options for bilateral intraocular retinoblastoma include the following:
- Enucleation for large intraocular tumors, followed by pathology-based, risk-adapted chemotherapy when the eye and vision cannot be saved.
- Conservative ocular salvage approaches when the eye and vision can be saved.
Intraocular tumor burden is usually asymmetric, and treatment is dictated by the most advanced eye. Systemic therapy is generally selected on the basis of the eye with more extensive disease. Treatment options described for unilateral disease may be applied to one or both affected eyes in patients with bilateral disease. While up-front enucleation of an advanced eye and risk-adapted adjuvant chemotherapy may be required, a more conservative approach using primary chemoreduction with close monitoring for response and aggressive local treatment is usually the treatment of choice. EBRT is now reserved for patients whose eyes do not respond adequately to primary systemic or intra-arterial chemotherapy and local consolidation.[ 19 ]
A number of large centers have published trial results that used systemic chemotherapy in conjunction with aggressive local consolidation for patients with bilateral disease.[ 20 ] The backbone of chemoreduction has generally been carboplatin, etoposide, and vincristine. While the less toxic combination of vincristine and carboplatin can provide disease control for a significant proportion of patients, ocular salvage appears to be superior when etoposide is included in the regimen.[ 21 ][Level of evidence: 1iiDiii] Similar results were achieved in one single-institution study when topotecan was substituted for etoposide in a combination regimen.[ 22 ][Level of evidence: 3iiA] Using this approach, the International Classification of Retinoblastoma grouping system has been proven to predict ocular survival, with globe salvage rates usually exceeding 80% for Groups A and B, and 40% to 80% for Groups C and D, although EBRT may be required in more advanced intraocular cases.[ 23 ]; [ 20 ][ 22 ][Level of evidence: 3iiA]
For patients with large intraocular tumor burdens with subretinal or vitreous seeds (Group D eyes), the administration of higher doses of carboplatin coupled with subtenon carboplatin, and the addition of lower doses of EBRT (36 Gy) for patients with persistent disease has been explored. Using this intensive approach, eye survival may approach a rate of 70% at 60 months.[ 24 ][Level of evidence: 2Div]
The use of prolonged systemic chemotherapy for Group E eyes to avoid or delay enucleation has been associated with lower disease-specific survival.[ 13 ][Level of evidence: 3iiiB]
Delivery of chemotherapy via ophthalmic artery cannulation with the addition of intra-vitreal chemotherapy for patients with persistent vitreous or subretinal disease has become a very strong alternative to the use of systemic chemotherapy (refer to the Ophthalmic Artery Infusion of Chemotherapy [Intra-arterial Chemotherapy] and Intravitreal Chemotherapy sections of this summary for more information).[ 14 ][ 15 ][ 17 ][ 18 ]; [ 16 ][Level of evidence: 3iiDiv] While tandem administration is feasible, bilateral administrations increase the risk of systemic toxicity caused by melphalan exposure.[ 25 ] In these circumstances, intra-arterial chemotherapy with single-agent carboplatin may be used to treat the less-advanced eye during the tandem procedure.[ 26 ] These treatments should only be performed in an experienced center with a state-of-the-art treatment infrastructure and a dedicated multidisciplinary team.
Treatment of Cavitary Retinoblastoma
Treatment options for cavitary retinoblastoma include the following:
- Systemic and/or intra-arterial chemotherapy.
In patients with cavitary retinoblastoma, minimal visual response is seen after intravenous chemotherapy and/or intra-arterial chemotherapy. Despite the blunted clinical response, cavitary retinoblastoma has a favorable long-term outcome, with stable tumor regression and globe salvage. Aggressive or prolonged chemotherapy or adjunctive therapies are generally not necessary. In a retrospective series of 26 cavitary retinoblastomas that were treated with intravenous chemoreduction and/or intra-arterial chemotherapy, the mean reduction in tumor base was 22%, and the mean reduction in tumor thickness was 29%. Despite minimal reduction, tumor recurrence was noted in only one eye, globe salvage was achieved in 22 eyes, and there were no cases of metastasis or death during 49 months (range, 6–189 months) of follow-up.[ 27 ]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
参考文献- Chantada GL, Guitter MR, Fandiño AC, et al.: Treatment results in patients with retinoblastoma and invasion to the cut end of the optic nerve. Pediatr Blood Cancer 52 (2): 218-22, 2009.[PUBMED Abstract]
- Eagle RC: High-risk features and tumor differentiation in retinoblastoma: a retrospective histopathologic study. Arch Pathol Lab Med 133 (8): 1203-9, 2009.[PUBMED Abstract]
- Aerts I, Sastre-Garau X, Savignoni A, et al.: Results of a multicenter prospective study on the postoperative treatment of unilateral retinoblastoma after primary enucleation. J Clin Oncol 31 (11): 1458-63, 2013.[PUBMED Abstract]
- Kaliki S, Shields CL, Rojanaporn D, et al.: High-risk retinoblastoma based on international classification of retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology 120 (5): 997-1003, 2013.[PUBMED Abstract]
- Sastre X, Chantada GL, Doz F, et al.: Proceedings of the consensus meetings from the International Retinoblastoma Staging Working Group on the pathology guidelines for the examination of enucleated eyes and evaluation of prognostic risk factors in retinoblastoma. Arch Pathol Lab Med 133 (8): 1199-202, 2009.[PUBMED Abstract]
- Chawla B, Sharma S, Sen S, et al.: Correlation between clinical features, magnetic resonance imaging, and histopathologic findings in retinoblastoma: a prospective study. Ophthalmology 119 (4): 850-6, 2012.[PUBMED Abstract]
- Laurent VE, Torbidoni AV, Sampor C, et al.: Minimal Disseminated Disease in Nonmetastatic Retinoblastoma With High-Risk Pathologic Features and Association With Disease-Free Survival. JAMA Ophthalmol 134 (12): 1374-1379, 2016.[PUBMED Abstract]
- Chantada GL, Dunkel IJ, de Dávila MT, et al.: Retinoblastoma patients with high risk ocular pathological features: who needs adjuvant therapy? Br J Ophthalmol 88 (8): 1069-73, 2004.[PUBMED Abstract]
- Cuenca A, Giron F, Castro D, et al.: Microscopic scleral invasion in retinoblastoma: clinicopathological features and outcome. Arch Ophthalmol 127 (8): 1006-10, 2009.[PUBMED Abstract]
- Chantada GL, Fandiño AC, Guitter MR, et al.: Results of a prospective study for the treatment of unilateral retinoblastoma. Pediatr Blood Cancer 55 (1): 60-6, 2010.[PUBMED Abstract]
- Shields CL, Honavar SG, Meadows AT, et al.: Chemoreduction plus focal therapy for retinoblastoma: factors predictive of need for treatment with external beam radiotherapy or enucleation. Am J Ophthalmol 133 (5): 657-64, 2002.[PUBMED Abstract]
- Shields CL, Honavar SG, Meadows AT, et al.: Chemoreduction for unilateral retinoblastoma. Arch Ophthalmol 120 (12): 1653-8, 2002.[PUBMED Abstract]
- Zhao J, Dimaras H, Massey C, et al.: Pre-enucleation chemotherapy for eyes severely affected by retinoblastoma masks risk of tumor extension and increases death from metastasis. J Clin Oncol 29 (7): 845-51, 2011.[PUBMED Abstract]
- Abramson DH, Fabius AW, Issa R, et al.: Advanced Unilateral Retinoblastoma: The Impact of Ophthalmic Artery Chemosurgery on Enucleation Rate and Patient Survival at MSKCC. PLoS One 10 (12): e0145436, 2015.[PUBMED Abstract]
- Munier FL, Mosimann P, Puccinelli F, et al.: First-line intra-arterial versus intravenous chemotherapy in unilateral sporadic group D retinoblastoma: evidence of better visual outcomes, ocular survival and shorter time to success with intra-arterial delivery from retrospective review of 20 years of treatment. Br J Ophthalmol 101 (8): 1086-1093, 2017.[PUBMED Abstract]
- Shields CL, Jorge R, Say EA, et al.: Unilateral Retinoblastoma Managed With Intravenous Chemotherapy Versus Intra-Arterial Chemotherapy. Outcomes Based on the International Classification of Retinoblastoma. Asia Pac J Ophthalmol (Phila) 5 (2): 97-103, 2016 Mar-Apr.[PUBMED Abstract]
- Francis JH, Iyer S, Gobin YP, et al.: Retinoblastoma Vitreous Seed Clouds (Class 3): A Comparison of Treatment with Ophthalmic Artery Chemosurgery with or without Intravitreous and Periocular Chemotherapy. Ophthalmology 124 (10): 1548-1555, 2017.[PUBMED Abstract]
- Francis JH, Levin AM, Zabor EC, et al.: Ten-year experience with ophthalmic artery chemosurgery: Ocular and recurrence-free survival. PLoS One 13 (5): e0197081, 2018.[PUBMED Abstract]
- Orman A, Koru-Sengul T, Miao F, et al.: The modern role of radiation therapy in treating advanced-stage retinoblastoma: long-term outcomes and racial differences. Int J Radiat Oncol Biol Phys 90 (5): 1037-43, 2014.[PUBMED Abstract]
- Rodriguez-Galindo C, Orbach DB, VanderVeen D: Retinoblastoma. Pediatr Clin North Am 62 (1): 201-23, 2015.[PUBMED Abstract]
- Lumbroso-Le Rouic L, Aerts I, Hajage D, et al.: Conservative treatment of retinoblastoma: a prospective phase II randomized trial of neoadjuvant chemotherapy followed by local treatments and chemothermotherapy. Eye (Lond) 30 (1): 46-52, 2016.[PUBMED Abstract]
- Brennan RC, Qaddoumi I, Mao S, et al.: Ocular Salvage and Vision Preservation Using a Topotecan-Based Regimen for Advanced Intraocular Retinoblastoma. J Clin Oncol 35 (1): 72-77, 2017.[PUBMED Abstract]
- Shields CL, Mashayekhi A, Au AK, et al.: The International Classification of Retinoblastoma predicts chemoreduction success. Ophthalmology 113 (12): 2276-80, 2006.[PUBMED Abstract]
- Berry JL, Jubran R, Kim JW, et al.: Long-term outcomes of Group D eyes in bilateral retinoblastoma patients treated with chemoreduction and low-dose IMRT salvage. Pediatr Blood Cancer 60 (4): 688-93, 2013.[PUBMED Abstract]
- Schaiquevich P, Buitrago E, Taich P, et al.: Pharmacokinetic analysis of melphalan after superselective ophthalmic artery infusion in preclinical models and retinoblastoma patients. Invest Ophthalmol Vis Sci 53 (7): 4205-12, 2012.[PUBMED Abstract]
- Francis JH, Gobin YP, Brodie SE, et al.: Experience of intra-arterial chemosurgery with single agent carboplatin for retinoblastoma. Br J Ophthalmol 96 (9): 1270-1, 2012.[PUBMED Abstract]
- Rojanaporn D, Kaliki S, Bianciotto CG, et al.: Intravenous chemoreduction or intra-arterial chemotherapy for cavitary retinoblastoma: long-term results. Arch Ophthalmol 130 (5): 585-90, 2012.[PUBMED Abstract]
- Treatment of Extraocular Retinoblastoma
-
In high-income countries, few patients with retinoblastoma present with extraocular disease. Extraocular disease may be localized to the soft tissues surrounding the eye or to the optic nerve beyond the margin of resection. However, further extension may progress into the brain and meninges, with subsequent seeding of the spinal fluid and as distant metastatic disease involving the lungs, bones, and bone marrow.
Treatment of Orbital and Locoregional Retinoblastoma
Orbital retinoblastoma occurs as a result of progression of the tumor through the emissary vessels and sclera. For this reason, transscleral disease is considered to be extraocular and should be treated as such. Orbital retinoblastoma is isolated in 60% to 70% of cases.
Treatment options for extraocular retinoblastoma (orbital and locoregional) include the following:
- Chemotherapy.
- Enucleation (for extraocular extension).
- Radiation therapy.
Treatment includes systemic chemotherapy and radiation therapy; with this approach, 60% to 85% of patients can be cured. Because most recurrences occur in the central nervous system (CNS), regimens that include drugs with well-documented CNS penetration are used. Different chemotherapy regimens have proven to be effective, including vincristine, cyclophosphamide, and doxorubicin and platinum- and epipodophyllotoxin-based regimens, or a combination of both.[ 1 ][ 2 ][ 3 ]
For patients with macroscopic orbital disease, delay of surgery until response to chemotherapy is achieved (usually after receiving two or three courses of treatment) has been effective. Patients then undergo enucleation and receive an additional four to six courses of chemotherapy. Next, local control is consolidated with orbital irradiation (40–45 Gy). Using this approach, orbital exenteration is not indicated.[ 3 ]
Patients with isolated involvement of the optic nerve at the transsection level are considered to have extraocular disease and are treated using systemic therapy, similar to that used for macroscopic orbital disease, and irradiation of the entire orbit (36 Gy) with a 10 Gy boost to the chiasm (total of 46 Gy).[ 2 ]
Treatment of CNS Disease
Intracranial dissemination occurs by direct extension through the optic nerve, and its prognosis is dismal. Treatment for these patients includes platinum-based, intensive systemic chemotherapy and CNS-directed therapy. Although intrathecal chemotherapy has been used traditionally, there is no preclinical or clinical evidence to support its use.
Treatment options for extraocular retinoblastoma (CNS disease) include the following:
- Systemic chemotherapy and CNS-directed therapy with radiation therapy.
- Systemic chemotherapy followed by myeloablative chemotherapy and stem cell rescue with or without radiation therapy.
The administration of radiation therapy to these patients is controversial. Responses have been observed with craniospinal radiation using 25 Gy to 35 Gy to the entire craniospinal axis and a boost (10 Gy) to sites of measurable disease.
Therapeutic intensification with high-dose, marrow-ablative chemotherapy and autologous hematopoietic progenitor cell rescue has been explored, but its role is not yet clear.[ 4 ][Level of evidence: 3iiA]
Treatment of Synchronous Trilateral Retinoblastoma
Trilateral retinoblastoma is usually associated with a pineal lesion or, less commonly, a suprasellar lesion.[ 5 ][ 6 ][ 7 ] In patients with the heritable form of retinoblastoma, CNS disease is less likely the result of metastatic or regional spread than of a primary intracranial focus, such as a pineal tumor. The prognosis for patients with trilateral retinoblastoma is very poor; most patients die of disseminated neuraxis disease in less than 9 months.[ 8 ][ 9 ] However, with increased surveillance and aggressive therapy, there has been improvement in survival, from 6% (patients treated before 1995) to 44% (patients treated after 1996).[ 10 ]
Treatment options for synchronous trilateral retinoblastoma include the following:
- Systemic chemotherapy followed by surgery and myeloablative chemotherapy with stem cell rescue.
- Systemic chemotherapy followed by surgery and radiation therapy.
While pineoblastomas occurring in older patients are sensitive to radiation therapy, current strategies are directed towards avoiding radiation by using intensive chemotherapy followed by consolidation with myeloablative chemotherapy and autologous hematopoietic progenitor cell rescue, an approach similar to those being used in the treatment of brain tumors in infants.[ 11 ]
(Refer to the Trilateral retinoblastoma section in the Causes of Retinoblastoma-Related Mortality section of this summary for more information about trilateral retinoblastoma, including screening with neuroimaging.)
Treatment of Extracranial Metastatic Retinoblastoma
Treatment options for extracranial metastatic retinoblastoma include the following:
- Systemic chemotherapy followed by myeloablative chemotherapy with stem cell rescue and radiation therapy.
Hematogenous metastases may develop in the bones, bone marrow and, less frequently, the liver. Although long-term survival has been reported with conventional chemotherapy, these reports should be considered anecdotal; metastatic retinoblastoma is not curable with conventional chemotherapy. In the last two decades, however, studies of small series of patients have shown that metastatic retinoblastoma can be cured using high-dose, marrow-ablative chemotherapy and autologous hematopoietic stem cell rescue.[ 12 ][ 13 ][ 14 ][ 15 ][ 16 ][ 17 ][ 18 ]; [ 19 ][Level of evidence: 3iiA]
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
参考文献- Antoneli CB, Ribeiro KB, Rodriguez-Galindo C, et al.: The addition of ifosfamide/etoposide to cisplatin/teniposide improves the survival of children with retinoblastoma and orbital involvement. J Pediatr Hematol Oncol 29 (10): 700-4, 2007.[PUBMED Abstract]
- Aerts I, Sastre-Garau X, Savignoni A, et al.: Results of a multicenter prospective study on the postoperative treatment of unilateral retinoblastoma after primary enucleation. J Clin Oncol 31 (11): 1458-63, 2013.[PUBMED Abstract]
- Radhakrishnan V, Kashyap S, Pushker N, et al.: Outcome, pathologic findings, and compliance in orbital retinoblastoma (International Retinoblastoma Staging System stage III) treated with neoadjuvant chemotherapy: a prospective study. Ophthalmology 119 (7): 1470-7, 2012.[PUBMED Abstract]
- Dunkel IJ, Chan HS, Jubran R, et al.: High-dose chemotherapy with autologous hematopoietic stem cell rescue for stage 4B retinoblastoma. Pediatr Blood Cancer 55 (1): 149-52, 2010.[PUBMED Abstract]
- Rodjan F, de Graaf P, Brisse HJ, et al.: Trilateral retinoblastoma: neuroimaging characteristics and value of routine brain screening on admission. J Neurooncol 109 (3): 535-44, 2012.[PUBMED Abstract]
- Paulino AC: Trilateral retinoblastoma: is the location of the intracranial tumor important? Cancer 86 (1): 135-41, 1999.[PUBMED Abstract]
- Blach LE, McCormick B, Abramson DH, et al.: Trilateral retinoblastoma--incidence and outcome: a decade of experience. Int J Radiat Oncol Biol Phys 29 (4): 729-33, 1994.[PUBMED Abstract]
- Kivelä T: Trilateral retinoblastoma: a meta-analysis of hereditary retinoblastoma associated with primary ectopic intracranial retinoblastoma. J Clin Oncol 17 (6): 1829-37, 1999.[PUBMED Abstract]
- Marcus DM, Brooks SE, Leff G, et al.: Trilateral retinoblastoma: insights into histogenesis and management. Surv Ophthalmol 43 (1): 59-70, 1998 Jul-Aug.[PUBMED Abstract]
- de Jong MC, Kors WA, de Graaf P, et al.: Trilateral retinoblastoma: a systematic review and meta-analysis. Lancet Oncol 15 (10): 1157-67, 2014.[PUBMED Abstract]
- Dunkel IJ, Jubran RF, Gururangan S, et al.: Trilateral retinoblastoma: potentially curable with intensive chemotherapy. Pediatr Blood Cancer 54 (3): 384-7, 2010.[PUBMED Abstract]
- Namouni F, Doz F, Tanguy ML, et al.: High-dose chemotherapy with carboplatin, etoposide and cyclophosphamide followed by a haematopoietic stem cell rescue in patients with high-risk retinoblastoma: a SFOP and SFGM study. Eur J Cancer 33 (14): 2368-75, 1997.[PUBMED Abstract]
- Kremens B, Wieland R, Reinhard H, et al.: High-dose chemotherapy with autologous stem cell rescue in children with retinoblastoma. Bone Marrow Transplant 31 (4): 281-4, 2003.[PUBMED Abstract]
- Rodriguez-Galindo C, Wilson MW, Haik BG, et al.: Treatment of metastatic retinoblastoma. Ophthalmology 110 (6): 1237-40, 2003.[PUBMED Abstract]
- Dunkel IJ, Aledo A, Kernan NA, et al.: Successful treatment of metastatic retinoblastoma. Cancer 89 (10): 2117-21, 2000.[PUBMED Abstract]
- Matsubara H, Makimoto A, Higa T, et al.: A multidisciplinary treatment strategy that includes high-dose chemotherapy for metastatic retinoblastoma without CNS involvement. Bone Marrow Transplant 35 (8): 763-6, 2005.[PUBMED Abstract]
- Jubran RF, Erdreich-Epstein A, Butturini A, et al.: Approaches to treatment for extraocular retinoblastoma: Children's Hospital Los Angeles experience. J Pediatr Hematol Oncol 26 (1): 31-4, 2004.[PUBMED Abstract]
- Palma J, Sasso DF, Dufort G, et al.: Successful treatment of metastatic retinoblastoma with high-dose chemotherapy and autologous stem cell rescue in South America. Bone Marrow Transplant 47 (4): 522-7, 2012.[PUBMED Abstract]
- Dunkel IJ, Khakoo Y, Kernan NA, et al.: Intensive multimodality therapy for patients with stage 4a metastatic retinoblastoma. Pediatr Blood Cancer 55 (1): 55-9, 2010.[PUBMED Abstract]
- Treatment of Progressive or Recurrent Retinoblastoma
-
The prognosis for a patient with progressive or recurrent retinoblastoma depends on the site and extent of the progression or recurrence and previous treatment received. Intraocular and extraocular recurrences have very different prognoses and are treated in distinctly different ways.
Treatment of Progressive or Recurrent Intraocular Retinoblastoma
Treatment options for progressive or recurrent intraocular retinoblastoma include the following:
- Enucleation.
- Radiation therapy (external-beam or plaque radiation therapy).
- Local treatments (cryotherapy or thermotherapy).
- Salvage chemotherapy (systemic or intra-arterial chemotherapy).
- Intravitreal chemotherapy, especially for refractory or recurrent vitreous seeding.
The use and potential applications of intra-arterial and intravitreal chemotherapy have been extensively discussed in previous sections of this summary (refer to the Ophthalmic Artery Infusion of Chemotherapy [Intra-arterial Chemotherapy] and Intravitreal Chemotherapy sections of this summary for more information).
New intraocular tumors can arise in patients with the heritable form of disease whose eyes have been treated with local control measures only, because every cell in the retina carries the RB1 mutation; this should not be considered a recurrence. Even with previous treatment consisting of chemoreduction and local control measures in very young patients with heritable retinoblastoma, surveillance may detect new tumors at an early stage, and additional local control therapy, including plaque radiation therapy, can be successful in eradicating the tumors.[ 1 ]
When the recurrence or progression of retinoblastoma is confined to the eye and is small, the prognosis for sight and survival may be excellent with local therapy only.[ 2 ][Level of evidence: 3iiDiv] If the recurrence or progression is confined to the eye but is extensive, the prognosis for sight is poor; however, survival remains excellent.
Intra-arterial chemotherapy into the ophthalmic artery has been effective in patients who relapse after systemic chemotherapy and radiation therapy.[ 3 ][ 4 ] Rescue intra-arterial chemotherapy has been used after primary intra-arterial chemotherapy. However, patients often require other treatment methods because of disease progression after second-line intra-arterial chemotherapy.[ 5 ] Radiation therapy should be considered for patients who have not been previously irradiated. Finally, enucleation may be required in cases of progressive disease after all eye-salvaging treatments have failed.
Treatment of Progressive or Recurrent Extraocular Retinoblastoma
Treatment options for progressive or recurrent extraocular retinoblastoma include the following:
- Systemic chemotherapy and radiation therapy for orbital disease.
- Systemic chemotherapy followed by myeloablative chemotherapy with stem cell rescue and radiation therapy for extraorbital disease.
Recurrence in the orbit after enucleation is treated with aggressive chemotherapy in addition to local radiation therapy because of the high risk of metastatic disease.[ 6 ][Level of evidence: 3iiA] After enucleation for recurrence, high-resolution magnetic resonance imaging with orbital coils can be helpful in distinguishing orbital recurrence from postsurgical enhancement.[ 7 ]
If the recurrence or progression is extraocular, the chance of survival is poor.[ 8 ] However, the use of intensive systemic chemotherapy and consolidation with high-dose chemotherapy and autologous hematopoietic stem cell rescue may improve the chance of a cure, particularly for patients with extracranial recurrence (refer to the Treatment of Extraocular Retinoblastoma section of this summary for more information). For patients with disease recurrence after those intensive approaches, clinical trials may be considered.
Treatment Options Under Clinical Evaluation for Progressive or Recurrent Retinoblastoma
One approach under investigation for patients with progressive intraocular retinoblastoma includes the use of an oncolytic adenovirus that targets RB1.[ 9 ]
Information about National Cancer Institute (NCI)–supported clinical trials can be found on the NCI website. For information about clinical trials sponsored by other organizations, refer to the ClinicalTrials.gov website.
The following is an example of a national and/or institutional clinical trial that is currently being conducted:
Current Clinical Trials
Use our advanced clinical trial search to find NCI-supported cancer clinical trials that are now enrolling patients. The search can be narrowed by location of the trial, type of treatment, name of the drug, and other criteria. General information about clinical trials is also available.
参考文献- Wilson MW, Haik BG, Billups CA, et al.: Incidence of new tumor formation in patients with hereditary retinoblastoma treated with primary systemic chemotherapy: is there a preventive effect? Ophthalmology 114 (11): 2077-82, 2007.[PUBMED Abstract]
- Chan MP, Hungerford JL, Kingston JE, et al.: Salvage external beam radiotherapy after failed primary chemotherapy for bilateral retinoblastoma: rate of eye and vision preservation. Br J Ophthalmol 93 (7): 891-4, 2009.[PUBMED Abstract]
- Schaiquevich P, Ceciliano A, Millan N, et al.: Intra-arterial chemotherapy is more effective than sequential periocular and intravenous chemotherapy as salvage treatment for relapsed retinoblastoma. Pediatr Blood Cancer 60 (5): 766-70, 2013.[PUBMED Abstract]
- Say EA, Iyer PG, Hasanreisoglu M, et al.: Secondary and tertiary intra-arterial chemotherapy for massive persistent or recurrent subretinal retinoblastoma seeds following previous chemotherapy exposure: long-term tumor control and globe salvage in 30 eyes. J AAPOS 20 (4): 337-42, 2016.[PUBMED Abstract]
- Francis JH, Abramson DH, Gobin YP, et al.: Efficacy and toxicity of second-course ophthalmic artery chemosurgery for retinoblastoma. Ophthalmology 122 (5): 1016-22, 2015.[PUBMED Abstract]
- Kim JW, Kathpalia V, Dunkel IJ, et al.: Orbital recurrence of retinoblastoma following enucleation. Br J Ophthalmol 93 (4): 463-7, 2009.[PUBMED Abstract]
- Sirin S, de Jong MC, de Graaf P, et al.: High-Resolution Magnetic Resonance Imaging Can Reliably Detect Orbital Tumor Recurrence after Enucleation in Children with Retinoblastoma. Ophthalmology 123 (3): 635-45, 2016.[PUBMED Abstract]
- Broaddus E, Topham A, Singh AD: Survival with retinoblastoma in the USA: 1975-2004. Br J Ophthalmol 93 (1): 24-7, 2009.[PUBMED Abstract]
- Pascual-Pasto G, Bazan-Peregrino M, Olaciregui NG, et al.: Therapeutic targeting of the RB1 pathway in retinoblastoma with the oncolytic adenovirus VCN-01. Sci Transl Med 11 (476): , 2019.[PUBMED Abstract]
- Changes to This Summary (12/03/2019)
-
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
This summary was reformatted.
General Information About Retinoblastoma
Added Table 1 about the pretest risk for relatives to carry the mutant RB1 allele of the proband.
Added Figure 2 about the management guidelines for childhood screening for retinoblastoma.
This summary is written and maintained by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® - NCI's Comprehensive Cancer Database pages.
- About This PDQ Summary
-
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the treatment of retinoblastoma. It is intended as a resource to inform and assist clinicians who care for cancer patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Pediatric Treatment Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Pediatric Treatment Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary].”
The preferred citation for this PDQ summary is:
PDQ® Pediatric Treatment Editorial Board. PDQ Retinoblastoma Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/retinoblastoma/hp/retinoblastoma-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389442]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
Based on the strength of the available evidence, treatment options may be described as either “standard” or “under clinical evaluation.” These classifications should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s Email Us.

 画像を拡大する
画像を拡大する