ご利用について
This PDQ cancer information summary has current information about the treatment of vulvar cancer. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Date Last Modified") is the date of the most recent change. The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board.
CONTENTS
- General Information About Vulvar Cancer
-
Vulvar cancer is a rare disease in which malignant (cancer) cells form in the tissues of the vulva.
Vulvar cancer forms in a woman's external genitalia. The vulva includes:

Anatomy of the vulva. The vulva includes the mons pubis, clitoris, inner and outer lips of the vagina, and the openings of the urethra and vagina. Vulvar cancer most often affects the outer vaginal lips. Less often, cancer affects the inner vaginal lips, clitoris, or vaginal glands.
Vulvar cancer usually forms slowly over many years. Abnormal cells can grow on the surface of the vulvar skin for a long time. This condition is called vulvar intraepithelial neoplasia (VIN). Because it is possible for VIN to become vulvar cancer, it is important to get treatment.
Having vulvar intraepithelial neoplasia or HPV infection can increase the risk of vulvar cancer.
Anything that increases your risk of getting a disease is called a risk factor. Having a risk factor does not mean that you will get cancer; not having risk factors doesn't mean that you will not get cancer. Talk with your doctor if you think you may be at risk. Risk factors for vulvar cancer include the following:
Other possible risk factors include the following:
Signs of vulvar cancer include bleeding or itching in the vulvar area.
Vulvar cancer often does not cause early signs or symptoms. Signs and symptoms may be caused by vulvar cancer or by other conditions. Check with your doctor if you have any of the following:
Tests that examine the vulva are used to diagnose vulvar cancer.
The following tests and procedures may be used:
Certain factors affect prognosis (chance of recovery) and treatment options.
The prognosis and treatment options depend on the following:
- Stages of Vulvar Cancer
-
After vulvar cancer has been diagnosed, tests are done to find out if cancer cells have spread within the vulva or to other parts of the body.
The process used to find out if cancer has spread within the vulva or to other parts of the body is called staging. The information gathered from the staging process determines the stage of the disease. It is important to know the stage in order to plan treatment. The following tests and procedures may be used in the staging process:
There are three ways that cancer spreads in the body.
Cancer can spread through tissue, the lymph system, and the blood:
Cancer may spread from where it began to other parts of the body.
When cancer spreads to another part of the body, it is called metastasis. Cancer cells break away from where they began (the primary tumor) and travel through the lymph system or blood.
The metastatic tumor is the same type of cancer as the primary tumor. For example, if vulvar cancer spreads to the lung, the cancer cells in the lung are actually vulvar cancer cells. The disease is metastatic vulvar cancer, not lung cancer.
In vulvar intraepithelial neoplasia (VIN), abnormal cells are found on the surface of the vulvar skin.
These abnormal cells are not cancer. Vulvar intraepithelial neoplasia (VIN) may become cancer and spread into nearby tissue. VIN is sometimes called stage 0 or carcinoma in situ.
The following stages are used for vulvar cancer:
Stage I
In stage I, cancer has formed. The tumor is found only in the vulva. Stage I is divided into stages IA and IB.
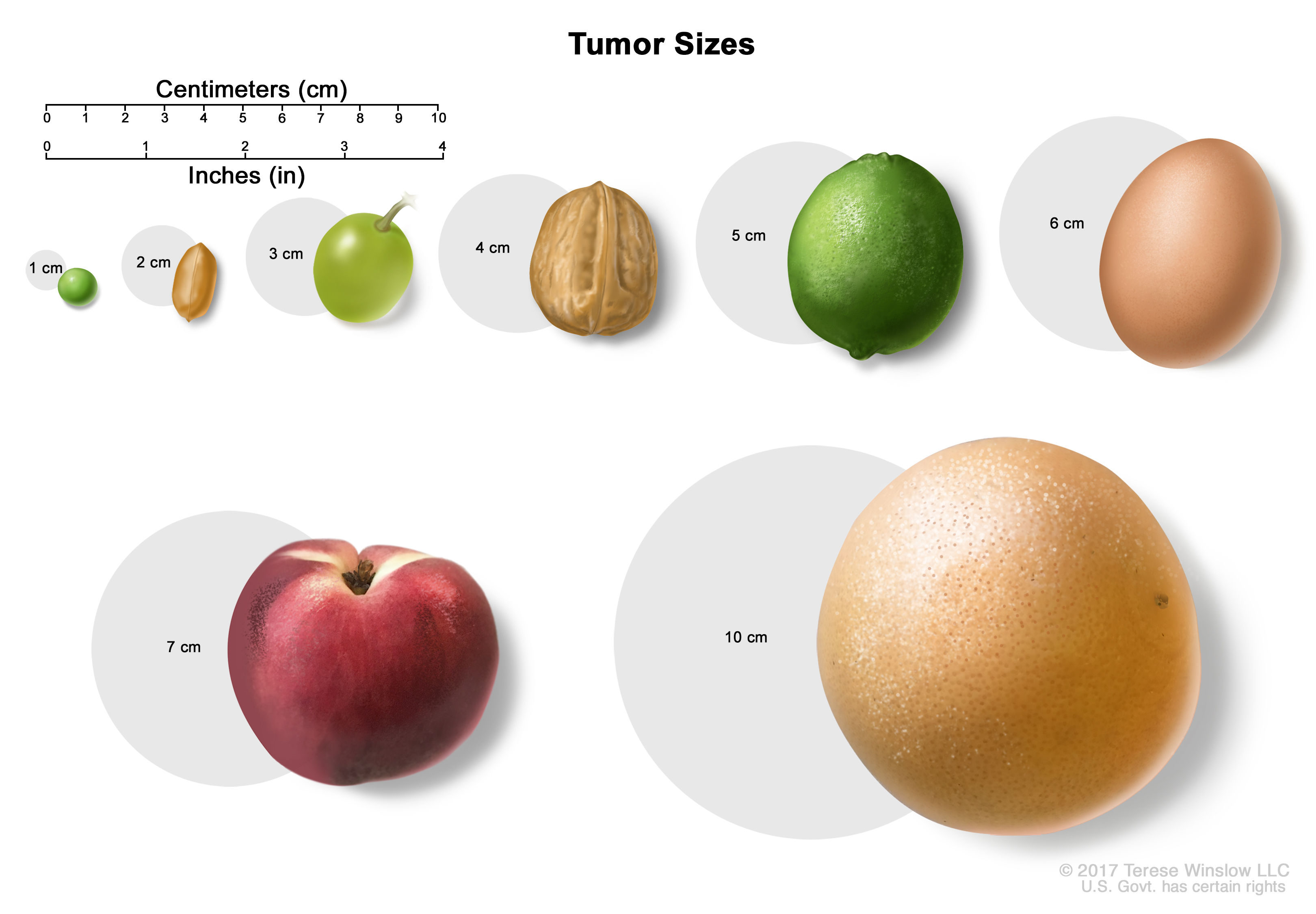
Tumor sizes are often measured in centimeters (cm) or inches. Common food items that can be used to show tumor size in cm include: a pea (1 cm), a peanut (2 cm), a grape (3 cm), a walnut (4 cm), a lime (5 cm or 2 inches), an egg (6 cm), a peach (7 cm), and a grapefruit (10 cm or 4 inches). Stage II
In stage II, the tumor is any size and has spread to the lower one-third of the urethra, the lower one-third of the vagina, or the lower one-third of the anus. Cancer has not spread to the lymph nodes.
Stage III
In stage III, the tumor is any size and has spread to the upper two-thirds of the urethra, the upper two-thirds of the vagina, the inner lining of the bladder or rectum, or to any number of lymph nodes. Stage III is divided into stages IIIA, IIIB, and IIIC.
Stage IV
In stage IV, the tumor is any size and has become attached to the bone, or cancer has spread to lymph nodes that are not movable or have become ulcerated, or there is distant spread. Stage IV is divided into stages IVA and IVB.
Vulvar cancer can recur (come back) after it has been treated.
The cancer may come back in the vulva or in other parts of the body.
- Treatment Option Overview
-
There are different types of treatment for patients with vulvar cancer.
Different types of treatments are available for patients with vulvar cancer. Some treatments are standard (the currently used treatment), and some are being tested in clinical trials. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment. Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Four types of standard treatment are used:
Surgery
Surgery is the most common treatment for vulvar intraepithelial neoplasia (VIN) and vulvar cancer.
One of the following types of surgery may be done to treat VIN:
The goal of surgery for vulvar cancer is to remove all the cancer without any loss of the woman's sexual function. One of the following types of surgery may be done to treat vulvar cancer:
After the doctor removes all the cancer that can be seen at the time of the surgery, some patients may be given chemotherapy and/or radiation therapy after surgery to kill any cancer cells that are left. Treatment given after the surgery, to lower the risk that the cancer will come back, is called adjuvant therapy.
Radiation therapy
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. External radiation therapy uses a machine outside the body to send radiation toward area of the body with cancer.
External radiation therapy may also be used as palliative therapy to relieve symptoms and improve quality of life.
Chemotherapy
Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping the cells from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy). Topical chemotherapy for vulvar cancer may be applied to the skin in a cream or lotion. The way the chemotherapy is given depends on the type and stage of the cancer being treated.
See Drugs Approved to Treat Vulvar Cancer for more information.
Immunotherapy
Immunotherapy is a treatment that uses the patient’s immune system to fight cancer. Substances made by the body or made in a laboratory are used to boost, direct, or restore the body’s natural defenses against cancer. This cancer treatment is a type of biologic therapy.
Imiquimod is an immune response modifier used to treat vulvar lesions and is applied to the skin in a cream.
New types of treatment are being tested in clinical trials.
Information about clinical trials is available from the NCI website.
Treatment for vulvar cancer may cause side effects.
For information about side effects caused by treatment for cancer, see our Side Effects page.
Patients may want to think about taking part in a clinical trial.
For some patients, taking part in a clinical trial may be the best treatment choice. Clinical trials are part of the cancer research process. Clinical trials are done to find out if new cancer treatments are safe and effective or better than the standard treatment.
Many of today's standard treatments for cancer are based on earlier clinical trials. Patients who take part in a clinical trial may receive the standard treatment or be among the first to receive a new treatment.
Patients who take part in clinical trials also help improve the way cancer will be treated in the future. Even when clinical trials do not lead to effective new treatments, they often answer important questions and help move research forward.
Patients can enter clinical trials before, during, or after starting their cancer treatment.
Some clinical trials only include patients who have not yet received treatment. Other trials test treatments for patients whose cancer has not gotten better. There are also clinical trials that test new ways to stop cancer from recurring (coming back) or reduce the side effects of cancer treatment.
Clinical trials are taking place in many parts of the country. Information about clinical trials supported by NCI can be found on NCI’s clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Follow-up tests may be needed.
Some of the tests that were done to diagnose the cancer or to find out the stage of the cancer may be repeated. Some tests will be repeated in order to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your condition has changed or if the cancer has recurred (come back). These tests are sometimes called follow-up tests or check-ups.
It is important to have regular follow-up exams to check for recurrent vulvar cancer.
- Treatment of Vulvar Intraepithelial Neoplasia (VIN)
-
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of vulvar intraepithelial neoplasia (VIN) may include the following:
-
Surgery may be one of the following:
- Separate excision of lesions.
- Wide local excision.
- Laser surgery.
- Ultrasound surgical aspiration.
- Skinning vulvectomy.
- Immunotherapy with topical imiquimod.
-
Surgery may be one of the following:
- Treatment of Stages I and II Vulvar Cancer
-
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of stage I vulvar cancer and stage II vulvar cancer may include the following:
- Surgery (wide local excision).
- Surgery (radical local excision with removal of lymph nodes in the groin and upper thigh).
- Surgery (modified radical vulvectomy or radical vulvectomy with removal of lymph nodes in the groin and upper thigh). Radiation therapy may be given.
- Surgery (radical local excision and removal of sentinel lymph node) followed by radiation therapy in some cases.
- Radiation therapy alone.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- Treatment of Stage III Vulvar Cancer
-
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of stage III vulvar cancer may include the following:
- Surgery (modified radical vulvectomy or radical vulvectomy with removal of lymph nodes in the groin and upper thigh) with or without radiation therapy.
- Radiation therapy or chemotherapy and radiation therapy followed by surgery.
- Radiation therapy with or without chemotherapy.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- Treatment of Stage IVA Vulvar Cancer
-
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of stage IVA vulvar cancer may include the following:
- Surgery (radical vulvectomy or pelvic exenteration).
- Surgery and radiation therapy.
- Radiation therapy or chemotherapy and radiation therapy followed by surgery.
- Radiation therapy with or without chemotherapy.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- Treatment of Stage IVB Vulvar Cancer
-
There is no standard treatment for stage IVB vulvar cancer. Chemotherapy has been studied and may be used if the patient can tolerate it.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- Treatment of Recurrent Vulvar Cancer
-
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of locally recurrent vulvar cancer may include the following:
- Surgery (wide local excision) with or without radiation therapy.
- Surgery (radical vulvectomy and pelvic exenteration).
- Chemotherapy and radiation therapy with or without surgery.
- Radiation therapy with or without chemotherapy.
- Radiation therapy and surgery.
- Radiation therapy as palliative treatment to relieve symptoms and improve quality of life.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- To Learn More About Vulvar Cancer
-
For more information from the National Cancer Institute about vulvar cancer, see the following:
For general cancer information and other resources from the National Cancer Institute, see the following:
- About This PDQ Summary
-
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about the treatment of vulvar cancer. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website. For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Vulvar Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/vulvar/patient/vulvar-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389324]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us.

 画像を拡大する
画像を拡大する