ご利用について
This PDQ cancer information summary has current information about the treatment of anal cancer. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Date Last Modified") is the date of the most recent change. The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board.
CONTENTS
- General Information About Anal Cancer
-
Anal cancer is a type of cancer that forms in the tissues of the anus.
The anus is the end of the large intestine. It is where stool (solid waste) leaves the body. The anus is formed partly from the outer skin layers of the body and partly from the intestine. The anus is connected to the rectum by the anal canal, which is about 1 to 1½ inches long. This area is controlled by two ring-like sphincter muscles, which contract to hold stool in and relax to allow its passage out of the body.

Anatomy of the lower gastrointestinal (digestive) system showing the colon, rectum, and anus. Other organs that make up the digestive system are also shown. Anal cancer can start in the lining of the anal canal, called the mucosa, or in the perianal skin, the squamous cells outside of the anus that contain hair follicles and sweat glands.
Tumors of the perianal skin that do not involve the anal sphincter are usually treated the same as anal cancers, although local therapy (treatment directed to a limited area of skin) may be used for some.
Most anal cancers are related to human papillomavirus (HPV) infection.
Risk factors for anal cancer include:
Signs of anal cancer include bleeding from the anus or rectum or a lump near the anus.
These and other signs and symptoms may be caused by anal cancer or by other conditions. Check with your doctor if you have:
Tests that examine the rectum and anus are used to diagnose anal cancer.
In addition to asking about your personal and family health history and doing a physical exam, your doctor may perform the following tests and procedures:
After anal cancer has been diagnosed, tests are done to find out if cancer cells have spread within the anus or to other parts of the body.
The process used to find out if cancer has spread within the anus or to other parts of the body is called staging. The information gathered from this staging process determines the stage of the disease. It is important to know the stage in order to plan treatment. The following tests may be used in the staging process:
Some people decide to get a second opinion.
You may want to get a second opinion to confirm your anal cancer diagnosis and treatment plan. If you seek a second opinion, you will need to get medical test results and reports from the first doctor to share with the second doctor. The second doctor will review the pathology report, slides, and scans. They may agree with the first doctor, suggest changes or another treatment approach, or provide more information about your cancer.
Learn more about choosing a doctor and getting a second opinion at Finding Cancer Care. You can contact NCI’s Cancer Information Service via chat, email, or phone (both in English and Spanish) for help finding a doctor, hospital, or getting a second opinion. For questions you might want to ask at your appointments, visit Questions to Ask Your Doctor About Cancer.
Certain factors affect the prognosis (chance of recovery) and treatment options.
The prognosis depends on:
The treatment options depend on:
- Stages of Anal Cancer
-
The following stages are used for anal cancer:
Stage 0 (carcinoma in situ)
In stage 0, abnormal cells are found in the mucosa (innermost layer) of the anus. These abnormal cells may become cancer and spread into nearby normal tissue. Stage 0 is also called high-grade intraepithelial lesion (HSIL).
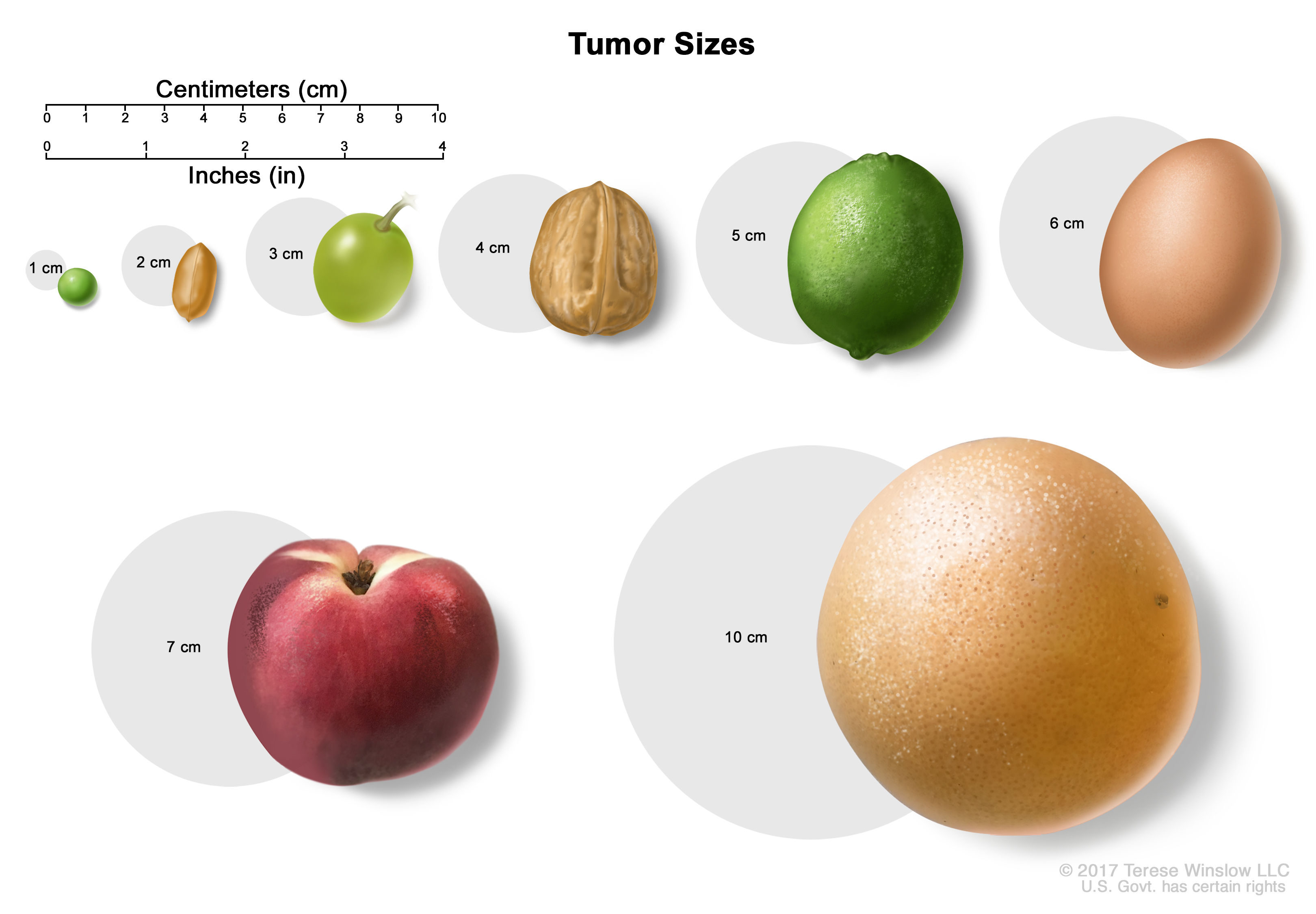
Tumor sizes are often measured in centimeters (cm) or inches. Common food items that can be used to show tumor size in cm include: a pea (1 cm), a peanut (2 cm), a grape (3 cm), a walnut (4 cm), a lime (5 cm or 2 inches), an egg (6 cm), a peach (7 cm), and a grapefruit (10 cm or 4 inches). Stage I (also called stage 1) anal cancer
In stage I, cancer has formed and the tumor is 2 centimeters or smaller.
Stage II (also called stage 2) anal cancer
Stage II anal cancer is divided into stages IIA and IIB.
Stage III (also called stage 3) anal cancer
Stage III anal cancer is divided into stages IIIA, IIIB, and IIIC.
Stage IV (also called stage 4) anal cancer
In stage IV, the tumor is any size. Cancer may have spread to lymph nodes or nearby organs and has spread to other parts of the body, such as the liver or lungs.
Stage IV anal cancer is also called metastatic anal cancer. Metastatic cancer happens when cancer cells travel through the lymphatic system or blood and form tumors in other parts of the body. The metastatic tumor is the same type of cancer as the primary tumor. For example, if anal cancer spreads to the liver, the cancer cells in the liver are actually anal cancer cells. The disease is called metastatic anal cancer, not liver cancer. Learn more in Metastatic Cancer: When Cancer Spreads.
Anal cancer can recur (come back) after it has been treated.
Recurrent anal cancer is cancer that has come back after it has been treated. If anal cancer comes back, it may come back in the anus or in other parts of the body, such as the liver or lungs. Tests will be done to help determine where the cancer has returned. The type of treatment for recurrent anal cancer will depend on where it has come back.
Learn more in Recurrent Cancer: When Cancer Comes Back. Information to help you cope and talk with your health care team can be found in the booklet When Cancer Returns.
- Treatment Option Overview
-
There are different types of treatment for people with anal cancer.
Different types of treatments are available for anal cancer. You and your cancer care team will work together to decide your treatment plan, which may include more than one type of treatment. Many factors will be considered, such as the stage of the cancer, your overall health, and your preferences. Your plan will include information about your cancer, the goals of treatment, your treatment options and the possible side effects, and the expected length of treatment.
Talking with your cancer care team before treatment begins about what to expect will be helpful. You’ll want to learn what you need to do before treatment begins, how you’ll feel while going through it, and what kind of help you will need. To learn more, visit Questions to Ask Your Doctor About Treatment.
The following types of treatment are used:
Surgery
Radiation therapy
Radiation therapy uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. There are two types of radiation therapy used to treat anal cancer:
Learn more about Radiation Therapy to Treat Cancer and Radiation Therapy Side Effects.
Chemotherapy
Chemotherapy (also called chemo) uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping the cells from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy).
Chemotherapy drugs used to treat anal cancer include:
Combinations of these drugs may be used. Other chemotherapy drugs not listed here may also be used.
Chemotherapy may be combined with other types of treatment, such as radiation therapy.
Learn more about how chemotherapy works, how it is given, common side effects, and more at Chemotherapy to Treat Cancer and Chemotherapy and You: Support for People With Cancer.
New types of treatment are being tested in clinical trials.
A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. For some patients, taking part in a clinical trial may be an option.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
To learn more about clinical trials, see Clinical Trials Information for Patients and Caregivers.
This summary section describes treatments that are being studied in clinical trials. It may not mention every new treatment being studied.
Radiosensitizers
Radiosensitizers are drugs that make tumor cells more sensitive to radiation therapy. Combining radiation therapy with radiosensitizers may kill more tumor cells.
Immunotherapy
Immunotherapy helps a person’s immune system fight cancer. Your doctor may suggest biomarker tests to help predict your response to certain immunotherapy drugs. Learn more about Biomarker Testing for Cancer.
Immunotherapy drugs used to treat anal cancer include:
Learn more about Immunotherapy to Treat Cancer.
Treatment for anal cancer may cause side effects.
For information about side effects caused by treatment for cancer, visit our Side Effects page.
Follow-up care may be needed.
As you go through treatment, you will have follow-up tests or check-ups. Some tests that were done to diagnose or stage the cancer may be repeated to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your condition has changed or if the cancer has recurred (come back).
- Treatment of Stage 0 (carcinoma in situ)
-
Treatment of stage 0 is usually local resection.
Learn more about this treatment in the Treatment Option Overview.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- Treatment of Stages I, II, and III Anal Cancer
-
Treatment of stage I, stage II, and stage III anal cancer may include:
Those who have had treatment that saves the sphincter muscles may receive follow-up exams every 3 months for the first 2 years, including rectal exams with endoscopy and biopsy, as needed to check for recurrence.
Learn more about these treatments in the Treatment Option Overview.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- Treatment of Stage IV Anal Cancer
-
Treatment of stage IV anal cancer may include:
Learn more about these treatments in the Treatment Option Overview.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- Treatment of HIV and Anal Cancer
In general, treatment for people who have anal cancer and HIV is similar to treatment for other people, and these patients have similar outcomes. However, this treatment can further damage the weakened immune systems of people who have HIV. Treatment for people with a history of AIDS-related complications may require lower doses of anticancer drugs and radiation therapy than doses used for patients who do not have HIV.
- Treatment of Recurrent Anal Cancer
-
Treatment of recurrent anal cancer may include:
Learn more about these treatments in the Treatment Option Overview.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- To Learn More About Anal Cancer
-
For more information from the National Cancer Institute about anal cancer, visit:
For general cancer information and other resources from the National Cancer Institute, visit:
- About This PDQ Summary
-
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about the treatment of anal cancer. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website. For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Anal Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/anal/patient/anal-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389368]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us.

 画像を拡大する
画像を拡大する