ご利用について
This PDQ cancer information summary has current information about the treatment of cervical cancer. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Date Last Modified") is the date of the most recent change. The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board.
CONTENTS
- General Information About Cervical Cancer
-
Cervical cancer is a disease in which malignant (cancer) cells form in the tissues of the cervix.
The cervix is the lower, narrow end of the uterus (the hollow, pear-shaped organ where a fetus grows). The cervix leads from the uterus to the vagina (birth canal).

Anatomy of the female reproductive system. The organs in the female reproductive system include the uterus, ovaries, fallopian tubes, cervix, and vagina. The uterus has a muscular outer layer called the myometrium and an inner lining called the endometrium. Cervical cancer usually develops slowly over time. Before cancer appears in the cervix, the cells of the cervix go through changes known as dysplasia, in which abnormal cells begin to appear in the cervical tissue. Over time, the abnormal cells may become cancer cells and start to grow and spread more deeply into the cervix and to surrounding areas.
Cervical cancer in children is rare.
See the following PDQ summaries for more information about cervical cancer:
Human papillomavirus (HPV) infection is the major risk factor for cervical cancer.
Anything that increases your chance of getting a disease is called a risk factor. Having a risk factor does not mean that you will get cancer; not having risk factors doesn't mean that you will not get cancer. Talk to your doctor if you think you may be at risk for cervical cancer.
Risk factors for cervical cancer include the following:
In women who are infected with HPV, the following risk factors add to the increased risk of cervical cancer:
There are also risk factors that increase the risk of HPV infection:
Older age is a main risk factor for most cancers. The chance of getting cancer increases as you get older.
There are usually no signs or symptoms of early cervical cancer but it can be detected early with regular check-ups.
Early cervical cancer may not cause signs or symptoms. Women should have regular check-ups, including tests to check for human papillomavirus (HPV) or abnormal cells in the cervix. The prognosis (chance of recovery) is better when the cancer is found early.
Signs and symptoms of cervical cancer include vaginal bleeding and pelvic pain.
These and other signs and symptoms may be caused by cervical cancer or by other conditions. Check with your doctor if you have any of the following:
Tests that examine the cervix are used to diagnose cervical cancer.
The following procedures may be used:
- Physical exam and health history: An exam of the body to check general signs of health, including checking for signs of disease, such as lumps or anything else that seems unusual. A history of the patient’s health habits and past illnesses and treatments will also be taken.
-
Pelvic exam: An exam of the vagina, cervix, uterus, fallopian tubes, ovaries, and rectum. A speculum is inserted into the vagina and the doctor or nurse looks at the vagina and cervix for signs of disease. A Pap test of the cervix is usually done. The doctor or nurse also inserts one or two lubricated, gloved fingers of one hand into the vagina and places the other hand over the lower abdomen to feel the size, shape, and position of the uterus and ovaries. The doctor or nurse also inserts a lubricated, gloved finger into the rectum to feel for lumps or abnormal areas.

Pelvic exam. A doctor or nurse inserts one or two lubricated, gloved fingers of one hand into the vagina and presses on the lower abdomen with the other hand. This is done to feel the size, shape, and position of the uterus and ovaries. The vagina, cervix, fallopian tubes, and rectum are also checked. -
Pap test: A procedure that uses a small brush to collect cells from the surface of the cervix and the area around it. The cells are viewed under a microscope to find out if they are abnormal. This procedure is also called a Pap smear.

Pap test. A speculum is inserted into the vagina to widen it. Then, a brush is inserted into the vagina to collect cells from the cervix. The cells are checked under a microscope for signs of disease. - Human papillomavirus (HPV) test: A laboratory test used to check DNA or RNA for certain types of HPV infection. Cells are collected from the cervix and DNA or RNA from the cells is checked to find out if an infection is caused by a type of HPV that is linked to cervical cancer. This test may be done using the sample of cells removed during a Pap test. This test may also be done if the results of a Pap test show certain abnormal cervical cells.
- Endocervical curettage: A procedure to collect cells or tissue from the cervical canal using a curette (spoon-shaped instrument). Tissue samples are taken and checked under a microscope for signs of cancer. This procedure is sometimes done at the same time as a colposcopy.
- Colposcopy: A procedure in which a colposcope (a lighted, magnifying instrument) is used to check the vagina and cervix for abnormal areas. Tissue samples may be taken using a curette (spoon-shaped instrument) or a brush and checked under a microscope for signs of disease.
- Biopsy: If abnormal cells are found in a Pap test, the doctor may do a biopsy. A sample of tissue is cut from the cervix and viewed under a microscope by a pathologist to check for signs of cancer. A biopsy that removes only a small amount of tissue is usually done in the doctor’s office. A woman may need to go to a hospital for a cervical cone biopsy (removal of a larger, cone-shaped sample of cervical tissue).
Certain factors affect prognosis (chance of recovery) and treatment options.
The prognosis depends on the following:
- The stage of the cancer (the size of the tumor and whether it affects part of the cervix or the whole cervix, or has spread to the lymph nodes or other places in the body).
- The type of cervical cancer.
- The patient's age and general health.
- Whether the patient has a certain type of human papillomavirus (HPV).
- Whether the patient has human immunodeficiency virus (HIV).
- Whether the cancer has just been diagnosed or has recurred (come back).
Treatment options depend on the following:
- The stage of the cancer.
- The type of cervical cancer.
- The patient's desire to have children.
- The patient’s age.
Treatment of cervical cancer during pregnancy depends on the stage of the cancer and the stage of the pregnancy. For cervical cancer found early or for cancer found during the last trimester of pregnancy, treatment may be delayed until after the baby is born. For more information, see the section on Cervical Cancer During Pregnancy.
- Stages of Cervical Cancer
-
After cervical cancer has been diagnosed, tests are done to find out if cancer cells have spread within the cervix or to other parts of the body.
The process used to find out if cancer has spread within the cervix or to other parts of the body is called staging. The information gathered from the staging process determines the stage of the disease. It is important to know the stage in order to plan treatment.
The following tests and procedures may be used in the staging process:
- CT scan (CAT scan): A procedure that makes a series of detailed pictures of areas inside the body, taken from different angles. The pictures are made by a computer linked to an x-ray machine. A dye may be injected into a vein or swallowed to help the organs or tissues show up more clearly. This procedure is also called computed tomography, computerized tomography, or computerized axial tomography.
- PET scan (positron emission tomography scan): A procedure to find malignant tumor cells in the body. A small amount of radioactive glucose (sugar) is injected into a vein. The PET scanner rotates around the body and makes a picture of where glucose is being used in the body. Malignant tumor cells show up brighter in the picture because they are more active and take up more glucose than normal cells do.
- MRI (magnetic resonance imaging): A procedure that uses a magnet, radio waves, and a computer to make a series of detailed pictures of areas inside the body. This procedure is also called nuclear magnetic resonance imaging (NMRI).
- Ultrasound exam: A procedure in which high-energy sound waves (ultrasound) are bounced off internal tissues or organs and make echoes. The echoes form a picture of body tissues called a sonogram. This picture can be printed to be looked at later.
- Chest x-ray: An x-ray of the organs and bones inside the chest. An x-ray is a type of energy beam that can go through the body and onto film, making a picture of areas inside the body.
- Lymph node biopsy: The removal of all or part of a lymph node. A pathologist views the lymph node tissue under a microscope to check for cancer cells.
- Cystoscopy: A procedure to look inside the bladder and urethra to check for abnormal areas. A cystoscope is inserted through the urethra into the bladder. A cystoscope is a thin, tube-like instrument with a light and a lens for viewing. It may also have a tool to remove tissue samples, which are checked under a microscope for signs of cancer.
- Laparoscopy: A surgical procedure to look at the organs inside the abdomen to check for signs of disease. Small incisions (cuts) are made in the wall of the abdomen and a laparoscope (a thin, lighted tube) is inserted into one of the incisions. Other instruments may be inserted through the same or other incisions to perform procedures such as removing organs or taking tissue samples to be checked under a microscope for signs of disease.
- Pretreatment surgical staging: Surgery (an operation) is done to find out if the cancer has spread within the cervix or to other parts of the body. In some cases, the cervical cancer can be removed at the same time. Pretreatment surgical staging is usually done only as part of a clinical trial.
The results of these tests are viewed together with the results of the original tumor biopsy to determine the cervical cancer stage.
There are three ways that cancer spreads in the body.
Cancer can spread through tissue, the lymph system, and the blood:
- Tissue. The cancer spreads from where it began by growing into nearby areas.
- Lymph system. The cancer spreads from where it began by getting into the lymph system. The cancer travels through the lymph vessels to other parts of the body.
- Blood. The cancer spreads from where it began by getting into the blood. The cancer travels through the blood vessels to other parts of the body.
Cancer may spread from where it began to other parts of the body.
When cancer spreads to another part of the body, it is called metastasis. Cancer cells break away from where they began (the primary tumor) and travel through the lymph system or blood.
- Lymph system. The cancer gets into the lymph system, travels through the lymph vessels, and forms a tumor (metastatic tumor) in another part of the body.
- Blood. The cancer gets into the blood, travels through the blood vessels, and forms a tumor (metastatic tumor) in another part of the body.
The metastatic tumor is the same type of cancer as the primary tumor. For example, if cervical cancer spreads to the lung, the cancer cells in the lung are actually cervical cancer cells. The disease is metastatic cervical cancer, not lung cancer.
Abnormal cells may form in the lining of the cervix (carcinoma in situ).
In carcinoma in situ, abnormal cells are found in the innermost lining of the cervix. These abnormal cells may become cancer and spread into nearby normal tissue.
The following stages are used for cervical cancer:
Stage I
In stage I, cancer has formed and is found in the cervix only.
Stage I is divided into stages IA and IB, based on the size of the tumor and the deepest point of tumor invasion.
- Stage IA: Stage IA is divided into stages IA1 and IA2, based on the deepest point of tumor invasion.

Stage IA1 and IA2 cervical cancer. A very small amount of cancer that can only be seen under a microscope is found in the tissues of the cervix. In stage IA1, the cancer is not more than 3 millimeters deep. In stage IA2, the cancer is more than 3 but not more than 5 millimeters deep. - In stage IA1, a very small amount of cancer that can only be seen with a microscope is found in the tissues of the cervix. The deepest point of tumor invasion is 3 millimeters or less.
- In stage IA2, a very small amount of cancer that can only be seen with a microscope is found in the tissues of the cervix. The deepest point of tumor invasion is more than 3 millimeters but not more than 5 millimeters.

Millimeters (mm). A sharp pencil point is about 1 mm, a new crayon point is about 2 mm, and a new pencil eraser is about 5 mm. - Stage IB: Stage IB is divided into stages IB1, IB2, and IB3, based on the size of the tumor and the deepest point of tumor invasion.
- In stage IB1, the tumor is 2 centimeters or smaller and the deepest point of tumor invasion is more than 5 millimeters.

Stage IB1 cervical cancer. The cancer is 2 centimeters or smaller and is more than 5 millimeters deep. - In stage IB2, the tumor is larger than 2 centimeters but not larger than 4 centimeters.

Stage IB2 and IB3 cervical cancer. In stage IB2, the cancer is larger than 2 centimeters but not larger than 4 centimeters. In stage IB3, the cancer is larger than 4 centimeters. - In stage IB3, the tumor is larger than 4 centimeters.
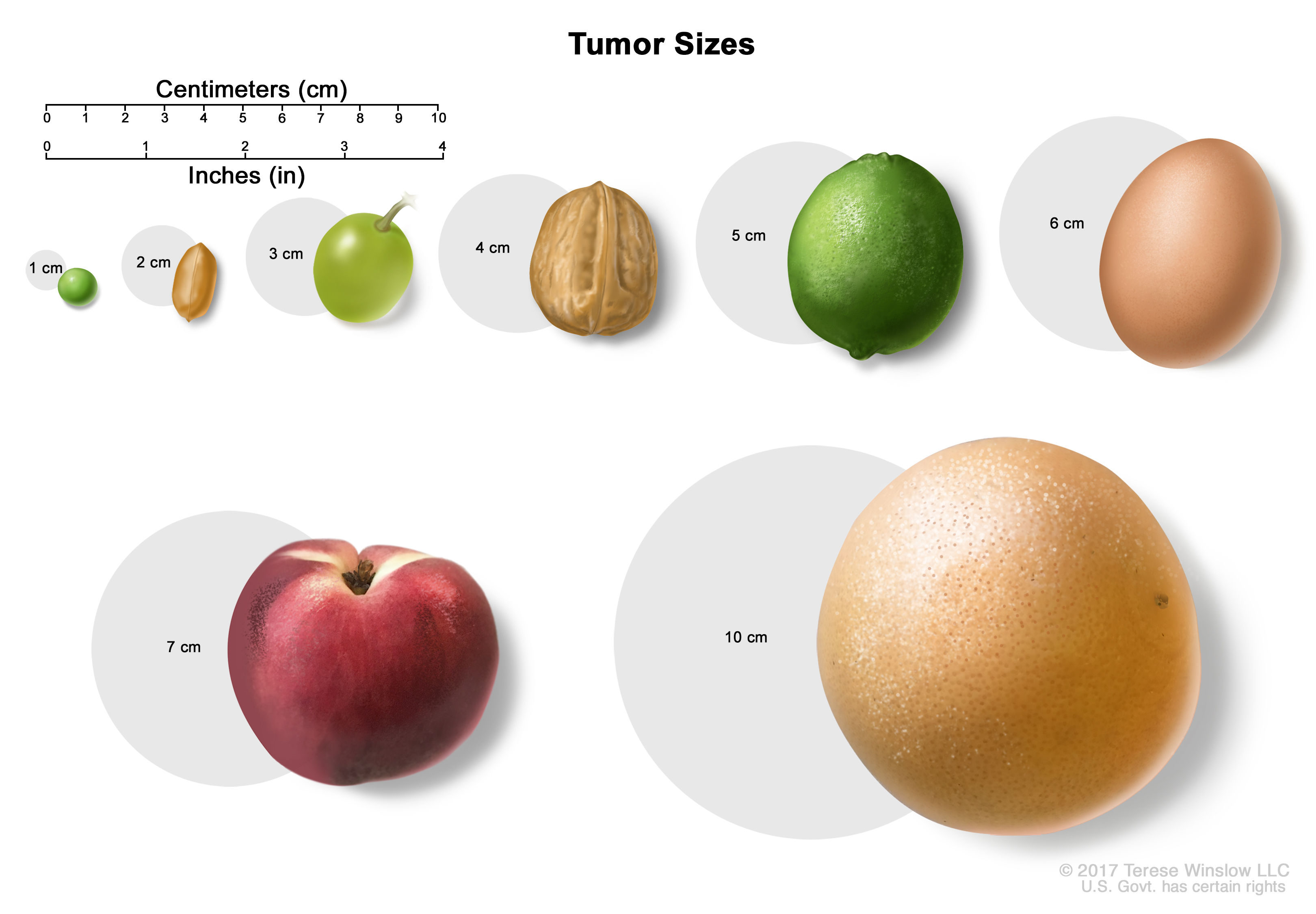
Tumor sizes are often measured in centimeters (cm) or inches. Common food items that can be used to show tumor size in cm include: a pea (1 cm), a peanut (2 cm), a grape (3 cm), a walnut (4 cm), a lime (5 cm or 2 inches), an egg (6 cm), a peach (7 cm), and a grapefruit (10 cm or 4 inches). - In stage IB1, the tumor is 2 centimeters or smaller and the deepest point of tumor invasion is more than 5 millimeters.
Stage II
In stage II, cancer has spread to the upper two-thirds of the vagina or to the tissue around the uterus.
Stage II is divided into stages IIA and IIB, based on how far the cancer has spread.

Stage II cervical cancer. In stages IIA1 and IIA2, cancer has spread from the cervix to the upper two-thirds of the vagina but has not spread to the tissue around the uterus. In stage IIA1, the cancer is 4 centimeters or smaller. In stage IIA2, the cancer is larger than 4 centimeters. In stage IIB, cancer has spread from the cervix to the tissue around the uterus. - Stage IIA: Cancer has spread from the cervix to the upper two-thirds of the vagina but has not spread to the tissue around the uterus. Stage IIA is divided into stages IIA1 and IIA2, based on the size of the tumor.
- In stage IIA1, the tumor is 4 centimeters or smaller.
- In stage IIA2, the tumor is larger than 4 centimeters.
- Stage IIB: Cancer has spread from the cervix to the tissue around the uterus.
Stage III
In stage III, cancer has spread to the lower third of the vagina and/or to the pelvic wall, and/or has caused kidney problems, and/or involves lymph nodes.
Stage III is divided into stages IIIA, IIIB, and IIIC, based on how far the cancer has spread.
- Stage IIIA: Cancer has spread to the lower third of the vagina but has not spread to the pelvic wall.

Stage IIIA cervical cancer. Cancer has spread to the lower third of the vagina but has not spread to the pelvic wall. - Stage IIIB: Cancer has spread to the pelvic wall; and/or the tumor has become large enough to block one or both ureters or has caused one or both kidneys to get bigger or stop working.

Stage IIIB cervical cancer. Cancer has spread to the pelvic wall and/or the tumor has become large enough to block one or both ureters or has caused one or both kidneys to get bigger or stop working. - Stage IIIC: Stage IIIC is divided into stages IIIC1 and IIIC2, based on the spread of cancer to the lymph nodes.

Stage IIIC cervical cancer. In stage IIIC1, cancer has spread to lymph nodes in the pelvis. In stage IIIC2, cancer has spread to lymph nodes in the abdomen near the aorta.
Stage IV
In stage IV, cancer has spread beyond the pelvis, or has spread to the lining of the bladder or rectum, or has spread to other parts of the body.
Stage IV is divided into stages IVA and IVB, based on where the cancer has spread.
- Stage IVA: Cancer has spread to nearby pelvic organs, such as the bladder or rectum.

Stage IVA cervical cancer. Cancer has spread to nearby pelvic organs, such as the bladder or rectum. - Stage IVB: Cancer has spread to other parts of the body, such as the liver, lungs, bones, or distant lymph nodes.

Stage IVB cervical cancer. Cancer has spread to other parts of the body, such as the lymph nodes, lung, liver, or bone.
Cervical cancer can recur (come back) after it has been treated.
The cancer may come back in the cervix or in other parts of the body.
- Treatment Option Overview
-
There are different types of treatment for patients with cervical cancer.
Different types of treatment are available for patients with cervical cancer. Some treatments are standard (the currently used treatment), and some are being tested in clinical trials. A treatment clinical trial is a research study meant to help improve current treatments or obtain information on new treatments for patients with cancer. When clinical trials show that a new treatment is better than the standard treatment, the new treatment may become the standard treatment. Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Five types of standard treatment are used:
Surgery
Surgery (removing the cancer in an operation) is sometimes used to treat cervical cancer. The following surgical procedures may be used:
-
Conization:
A procedure to remove a cone-shaped piece of tissue from the cervix and cervical canal. A pathologist views the tissue under a microscope to look for cancer cells. Conization may be used to diagnose or treat a cervical condition. This procedure is also called a cone biopsy.
Conization may be done using one of the following procedures:
- Cold-knife conization: A surgical procedure that uses a scalpel (sharp knife) to remove abnormal tissue or cancer.
- Loop electrosurgical excision procedure (LEEP): A surgical procedure that uses electrical current passed through a thin wire loop as a knife to remove abnormal tissue or cancer.
- Laser surgery: A surgical procedure that uses a laser beam (a narrow beam of intense light) as a knife to make bloodless cuts in tissue or to remove a surface lesion such as a tumor.
The type of conization procedure used depends on where the cancer cells are in the cervix and the type of cervical cancer.
- Total hysterectomy: Surgery to remove the uterus, including the cervix. If the uterus and cervix are taken out through the vagina, the operation is called a vaginal hysterectomy. If the uterus and cervix are taken out through a large incision (cut) in the abdomen, the operation is called a total abdominal hysterectomy. If the uterus and cervix are taken out through a small incision in the abdomen using a laparoscope, the operation is called a total laparoscopic hysterectomy.

Hysterectomy. The uterus is surgically removed with or without other organs or tissues. In a total hysterectomy, the uterus and cervix are removed. In a total hysterectomy with salpingo-oophorectomy, (a) the uterus plus one (unilateral) ovary and fallopian tube are removed; or (b) the uterus plus both (bilateral) ovaries and fallopian tubes are removed. In a radical hysterectomy, the uterus, cervix, both ovaries, both fallopian tubes, and nearby tissue are removed. These procedures are done using a low transverse incision or a vertical incision. - Radical hysterectomy: Surgery to remove the uterus, cervix, part of the vagina, and a wide area of ligaments and tissues around these organs. The ovaries, fallopian tubes, or nearby lymph nodes may also be removed.
- Modified radical hysterectomy: Surgery to remove the uterus, cervix, upper part of the vagina, and ligaments and tissues that closely surround these organs. Nearby lymph nodes may also be removed. In this type of surgery, not as many tissues and/or organs are removed as in a radical hysterectomy.
- Radical trachelectomy: Surgery to remove the cervix, nearby tissue and lymph nodes, and the upper part of the vagina. The uterus and ovaries are not removed.
- Bilateral salpingo-oophorectomy: Surgery to remove both ovaries and both fallopian tubes.
- Pelvic exenteration: Surgery to remove the lower colon, rectum, and bladder. The cervix, vagina, ovaries, and nearby lymph nodes are also removed. Artificial openings (stoma) are made for urine and stool to flow from the body to a collection bag. Plastic surgery may be needed to make an artificial vagina after this operation.
Radiation therapy
Radiation therapy is a cancer treatment that uses high-energy x-rays or other types of radiation to kill cancer cells or keep them from growing. There are two types of radiation therapy:
-
External radiation therapy uses a machine outside the body to send radiation toward the area of the body with cancer. Certain ways of giving radiation therapy can help keep radiation from damaging nearby healthy tissue. This type of radiation therapy includes the following:
- Intensity-modulated radiation therapy (IMRT): IMRT is a type of 3-dimensional (3-D) radiation therapy that uses a computer to make pictures of the size and shape of the tumor. Thin beams of radiation of different intensities (strengths) are aimed at the tumor from many angles.
- Internal radiation therapy uses a radioactive substance sealed in needles, seeds, wires, or catheters that are placed directly into or near the cancer.
The way the radiation therapy is given depends on the type and stage of the cancer being treated. External and internal radiation therapy are used to treat cervical cancer, and may also be used as palliative therapy to relieve symptoms and improve quality of life.
Chemotherapy
Chemotherapy is a cancer treatment that uses drugs to stop the growth of cancer cells, either by killing the cells or by stopping them from dividing. When chemotherapy is taken by mouth or injected into a vein or muscle, the drugs enter the bloodstream and can reach cancer cells throughout the body (systemic chemotherapy).
See Drugs Approved for Cervical Cancer for more information.
Targeted therapy
Targeted therapy is a type of treatment that uses drugs or other substances to identify and attack specific cancer cells. Targeted therapies usually cause less harm to normal cells than chemotherapy or radiation therapy do.
Monoclonal antibody therapy is a type of targeted therapy.
- Monoclonal antibodies: Monoclonal antibodies are immune system proteins made in the laboratory to treat many diseases, including cancer. As a cancer treatment, these antibodies can attach to a specific target on cancer cells or other cells that may help cancer cells grow. The antibodies are able to then kill the cancer cells, block their growth, or keep them from spreading. Monoclonal antibodies are given by infusion. They may be used alone or to carry drugs, toxins, or radioactive material directly to cancer cells.
Bevacizumab is a monoclonal antibody that binds to a protein called vascular endothelial growth factor (VEGF) and may prevent the growth of new blood vessels that tumors need to grow. Bevacizumab is used to treat cervical cancer that has metastasized (spread to other parts of the body) and recurrent cervical cancer.
See Drugs Approved for Cervical Cancer for more information.
Immunotherapy
Immunotherapy is a treatment that uses the patient's immune system to fight cancer. Substances made by the body or made in a laboratory are used to boost, direct, or restore the body's natural defenses against cancer. This cancer treatment is a type of biologic therapy.
Immune checkpoint inhibitor therapy is a type of immunotherapy.
- PD-1 and PD-L1 inhibitor therapy: PD-1 is a protein on the surface of T cells that helps keep the body’s immune responses in check. PD-L1 is a protein found on some types of cancer cells. When PD-1 attaches to PD-L1, it stops the T cell from killing the cancer cell. PD-1 and PD-L1 inhibitors keep PD-1 and PD-L1 proteins from attaching to each other. This allows the T cells to kill cancer cells. Pembrolizumab is a type of PD-1 inhibitor.

Immune checkpoint inhibitor. Checkpoint proteins, such as PD-L1 on tumor cells and PD-1 on T cells, help keep immune responses in check. The binding of PD-L1 to PD-1 keeps T cells from killing tumor cells in the body (left panel). Blocking the binding of PD-L1 to PD-1 with an immune checkpoint inhibitor (anti-PD-L1 or anti-PD-1) allows the T cells to kill tumor cells (right panel). See Drugs Approved for Cervical Cancer for more information.
New types of treatment are being tested in clinical trials.
Information about clinical trials is available from the NCI website.
Treatment for cervical cancer may cause side effects.
For information about side effects caused by treatment for cancer, see our Side Effects page.
Patients may want to think about taking part in a clinical trial.
For some patients, taking part in a clinical trial may be the best treatment choice. Clinical trials are part of the cancer research process. Clinical trials are done to find out if new cancer treatments are safe and effective or better than the standard treatment.
Many of today's standard treatments for cancer are based on earlier clinical trials. Patients who take part in a clinical trial may receive the standard treatment or be among the first to receive a new treatment.
Patients who take part in clinical trials also help improve the way cancer will be treated in the future. Even when clinical trials do not lead to effective new treatments, they often answer important questions and help move research forward.
Patients can enter clinical trials before, during, or after starting their cancer treatment.
Some clinical trials only include patients who have not yet received treatment. Other trials test treatments for patients whose cancer has not gotten better. There are also clinical trials that test new ways to stop cancer from recurring (coming back) or reduce the side effects of cancer treatment.
Clinical trials are taking place in many parts of the country. Information about clinical trials supported by NCI can be found on NCI’s clinical trials search webpage. Clinical trials supported by other organizations can be found on the ClinicalTrials.gov website.
Follow-up tests may be needed.
Some of the tests that were done to diagnose the cancer or to find out the stage of the cancer may be repeated. Some tests will be repeated in order to see how well the treatment is working. Decisions about whether to continue, change, or stop treatment may be based on the results of these tests.
Some of the tests will continue to be done from time to time after treatment has ended. The results of these tests can show if your condition has changed or if the cancer has recurred (come back). These tests are sometimes called follow-up tests or check-ups.
Your doctor will ask if you have any of the following signs or symptoms, which may mean the cancer has come back:
- Pain in the abdomen, back, or leg.
- Swelling in the leg.
- Trouble urinating.
- Cough.
- Feeling tired.
For cervical cancer, follow-up tests are usually done every 3 to 4 months for the first 2 years, followed by check-ups every 6 months. The check-up includes a current health history and exam of the body to check for signs and symptoms of recurrent cervical cancer and for late effects of treatment.
-
Conization:
A procedure to remove a cone-shaped piece of tissue from the cervix and cervical canal. A pathologist views the tissue under a microscope to look for cancer cells. Conization may be used to diagnose or treat a cervical condition. This procedure is also called a cone biopsy.
- Treatment of Carcinoma in Situ
-
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of carcinoma in situ may include the following:
- Conization, such as cold-knife conization, loop electrosurgical excision procedure (LEEP), or laser surgery.
- Hysterectomy for women who cannot or no longer want to have children. This is done only if the tumor cannot be completely removed by conization.
- Internal radiation therapy for women who cannot have surgery.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- Treatment of Stage IA Cervical Cancer
-
For information about the treatments listed below, see the Treatment Option Overview section.
Stage IA cervical cancer is separated into stage IA1 and IA2.
Treatment for stage IA1 may include the following:
- Conization.
- Total hysterectomy with or without bilateral salpingo-oophorectomy.
Treatment for stage IA2 may include the following:
- Modified radical hysterectomy and removal of lymph nodes.
- Radical trachelectomy.
- Internal radiation therapy for women who cannot have surgery.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- Treatment of Stages IB and IIA Cervical Cancer
-
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of stage IB and stage IIA cervical cancer may include the following:
- Radiation therapy with chemotherapy given at the same time.
- Radical hysterectomy and removal of pelvic lymph nodes with or without radiation therapy to the pelvis, plus chemotherapy.
- Radical trachelectomy.
- Chemotherapy followed by surgery.
- Radiation therapy alone.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- Treatment of Stages IIB, III, and IVA Cervical Cancer
-
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of stage IIB, stage III, and stage IVA cervical cancer may include the following:
- Radiation therapy with chemotherapy given at the same time.
- Surgery to remove pelvic lymph nodes followed by radiation therapy with or without chemotherapy.
- Internal radiation therapy.
- A clinical trial of chemotherapy to shrink the tumor followed by surgery.
- A clinical trial of chemotherapy and radiation therapy given at the same time, followed by chemotherapy.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- Treatment of Stage IVB Cervical Cancer
-
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of stage IVB cervical cancer may include the following:
- Radiation therapy as palliative therapy to relieve symptoms caused by the cancer and improve quality of life.
- Chemotherapy and targeted therapy.
- Chemotherapy as palliative therapy to relieve symptoms caused by the cancer and improve quality of life.
- Clinical trials of new anticancer drugs or drug combinations.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- Treatment of Recurrent Cervical Cancer
-
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment of recurrent cervical cancer may include the following:
- Immunotherapy.
- Radiation therapy and chemotherapy.
- Chemotherapy and targeted therapy.
- Chemotherapy as palliative therapy to relieve symptoms caused by the cancer and improve quality of life.
- Pelvic exenteration.
- Clinical trials of new anticancer drugs or drug combinations.
Use our clinical trial search to find NCI-supported cancer clinical trials that are accepting patients. You can search for trials based on the type of cancer, the age of the patient, and where the trials are being done. General information about clinical trials is also available.
- Cervical Cancer During Pregnancy
-
General Information About Cervical Cancer During Pregnancy
Treatment of cervical cancer during pregnancy depends on the stage of the cancer and how long the patient has been pregnant. A biopsy and imaging tests may be done to determine the stage of the disease. To avoid exposing the fetus to radiation, MRI (magnetic resonance imaging) is used.
Treatment of Carcinoma in Situ During Pregnancy
Usually, no treatment is needed for carcinoma in situ during pregnancy. A colposcopy may be done to check for invasive cancer.
Treatment of Stage I Cervical Cancer During Pregnancy
For information about the treatments listed below, see the Treatment Option Overview section.
Pregnant women with slow-growing stage I cervical cancer may be able to delay treatment until the second trimester of pregnancy or after delivery.
Pregnant women with fast-growing stage I cervical cancer may need immediate treatment. Treatment may include:
Women should be tested to find out if the cancer has spread to the lymph nodes. If cancer has spread to the lymph nodes, immediate treatment may be needed.
Treatment of Stages II, III, and IV Cervical Cancer During Pregnancy
For information about the treatments listed below, see the Treatment Option Overview section.
Treatment for stage II, stage III, and stage IV cervical cancer during pregnancy may include the following:
- Chemotherapy to shrink the tumor in the second or third trimester of pregnancy. Surgery or radiation therapy may be done after delivery.
- Radiation therapy plus chemotherapy. Talk with your doctor about the effects of radiation on the fetus. It may be necessary to end the pregnancy before treatment begins.
- To Learn More About Cervical Cancer
-
For more information from the National Cancer Institute about cervical cancer, see the following:
- Cervical Cancer Home Page
- Cervical Cancer Prevention
- Cervical Cancer Screening
- Childhood Cervical and Vaginal Cancer Treatment
- Drugs Approved for Cervical Cancer
- Lasers to Treat Cancer
- Understanding Cervical Changes: A Health Guide for Women
- Human Papillomavirus (HPV) Vaccines
- HPV and Pap Testing
- Targeted Cancer Therapies
- Immunotherapy to Treat Cancer
For general cancer information and other resources from the National Cancer Institute, see the following:
- About This PDQ Summary
-
About PDQ
Physician Data Query (PDQ) is the National Cancer Institute's (NCI's) comprehensive cancer information database. The PDQ database contains summaries of the latest published information on cancer prevention, detection, genetics, treatment, supportive care, and complementary and alternative medicine. Most summaries come in two versions. The health professional versions have detailed information written in technical language. The patient versions are written in easy-to-understand, nontechnical language. Both versions have cancer information that is accurate and up to date and most versions are also available in Spanish.
PDQ is a service of the NCI. The NCI is part of the National Institutes of Health (NIH). NIH is the federal government’s center of biomedical research. The PDQ summaries are based on an independent review of the medical literature. They are not policy statements of the NCI or the NIH.
Purpose of This Summary
This PDQ cancer information summary has current information about the treatment of cervical cancer. It is meant to inform and help patients, families, and caregivers. It does not give formal guidelines or recommendations for making decisions about health care.
Reviewers and Updates
Editorial Boards write the PDQ cancer information summaries and keep them up to date. These Boards are made up of experts in cancer treatment and other specialties related to cancer. The summaries are reviewed regularly and changes are made when there is new information. The date on each summary ("Updated") is the date of the most recent change.
The information in this patient summary was taken from the health professional version, which is reviewed regularly and updated as needed, by the PDQ Adult Treatment Editorial Board.
Clinical Trial Information
A clinical trial is a study to answer a scientific question, such as whether one treatment is better than another. Trials are based on past studies and what has been learned in the laboratory. Each trial answers certain scientific questions in order to find new and better ways to help cancer patients. During treatment clinical trials, information is collected about the effects of a new treatment and how well it works. If a clinical trial shows that a new treatment is better than one currently being used, the new treatment may become "standard." Patients may want to think about taking part in a clinical trial. Some clinical trials are open only to patients who have not started treatment.
Clinical trials can be found online at NCI's website. For more information, call the Cancer Information Service (CIS), NCI's contact center, at 1-800-4-CANCER (1-800-422-6237).
Permission to Use This Summary
PDQ is a registered trademark. The content of PDQ documents can be used freely as text. It cannot be identified as an NCI PDQ cancer information summary unless the whole summary is shown and it is updated regularly. However, a user would be allowed to write a sentence such as “NCI’s PDQ cancer information summary about breast cancer prevention states the risks in the following way: [include excerpt from the summary].”
The best way to cite this PDQ summary is:
PDQ® Adult Treatment Editorial Board. PDQ Cervical Cancer Treatment. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/types/cervical/patient/cervical-treatment-pdq. Accessed <MM/DD/YYYY>. [PMID: 26389422]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use in the PDQ summaries only. If you want to use an image from a PDQ summary and you are not using the whole summary, you must get permission from the owner. It cannot be given by the National Cancer Institute. Information about using the images in this summary, along with many other images related to cancer can be found in Visuals Online. Visuals Online is a collection of more than 3,000 scientific images.
Disclaimer
The information in these summaries should not be used to make decisions about insurance reimbursement. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website’s E-mail Us.

 画像を拡大する
画像を拡大する